
Am Fam Physician. 2021;104(2):186-192
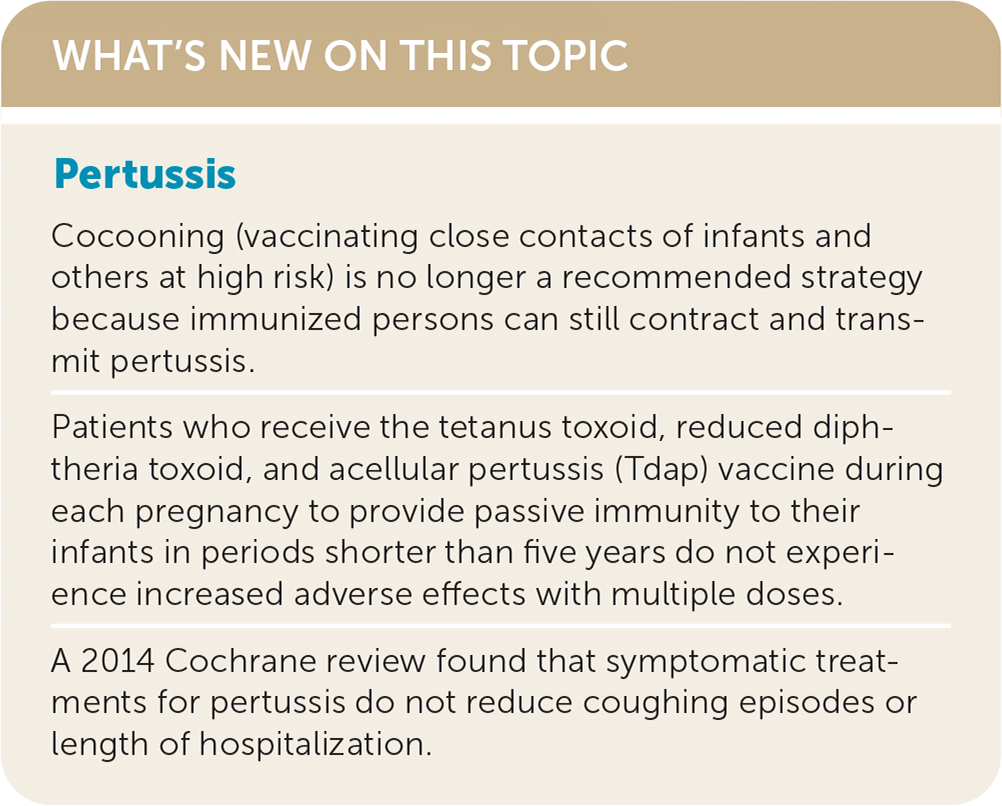
Pertussis, also known as whooping cough, remains a public health concern despite expanded immunization recommendations over the past three decades. The presentation of pertussis, which is variable and evolves over the course of the disease, includes nonspecific symptoms in the catarrhal stage, coughing with the classic whooping in the paroxysmal stage, and persistent cough in the convalescent stage. When there is clinical suspicion for pertussis, the diagnosis should be confirmed using polymerase chain reaction testing, which has replaced culture as the preferred confirmatory test. Recent evidence has confirmed a waning of acquired immunity following pertussis immunization or infection, leading to changes in tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis (Tdap) immunization recommendations. Patients 11 years or older should receive at least one dose of Tdap, although Tdap may replace any dose of the tetanus and diphtheria toxoids (Td) vaccine. All pregnant patients should receive Tdap between 27 and 36 weeks' gestation with each pregnancy to convey immunity to the newborn. Cocooning (vaccinating close contacts of high-risk individuals) is no longer recommended because immunized patients can still contract and transmit pertussis. A history of seizure or hypotonic-hyporesponsive episodes after a prior pertussis vaccination is no longer a contraindication to immunization. Antibiotic treatment is intended to prevent transmission of pertussis to others and does not shorten the disease course or improve symptoms. Antibiotic prophylaxis is recommended for household contacts of someone with pertussis and for those exposed to pertussis who are at high risk of severe illness (e.g., infants, people who are immunocompromised or in the third trimester of pregnancy) or in close contact with someone at high risk. Azithromycin is the preferred antibiotic for treatment or prophylaxis.
Pertussis, or whooping cough, is an acute respiratory tract infection that continues to affect a significant portion of the global population, with more than 24 million estimated cases in 2014.1 Pertussis, a Centers for Disease Control and Prevention (CDC) reportable disease, is caused by Bordetella pertussis. The disease can lead to substantial complications in infants, such as apnea, pneumonia, seizures, other hypoxic complications, hospitalization, or death.2,3 Bordetella parapertussis and rarely Bordetella bronchiseptica can also cause a pertussis-like syndrome.
What Is the Typical Presentation and Progression of Pertussis?
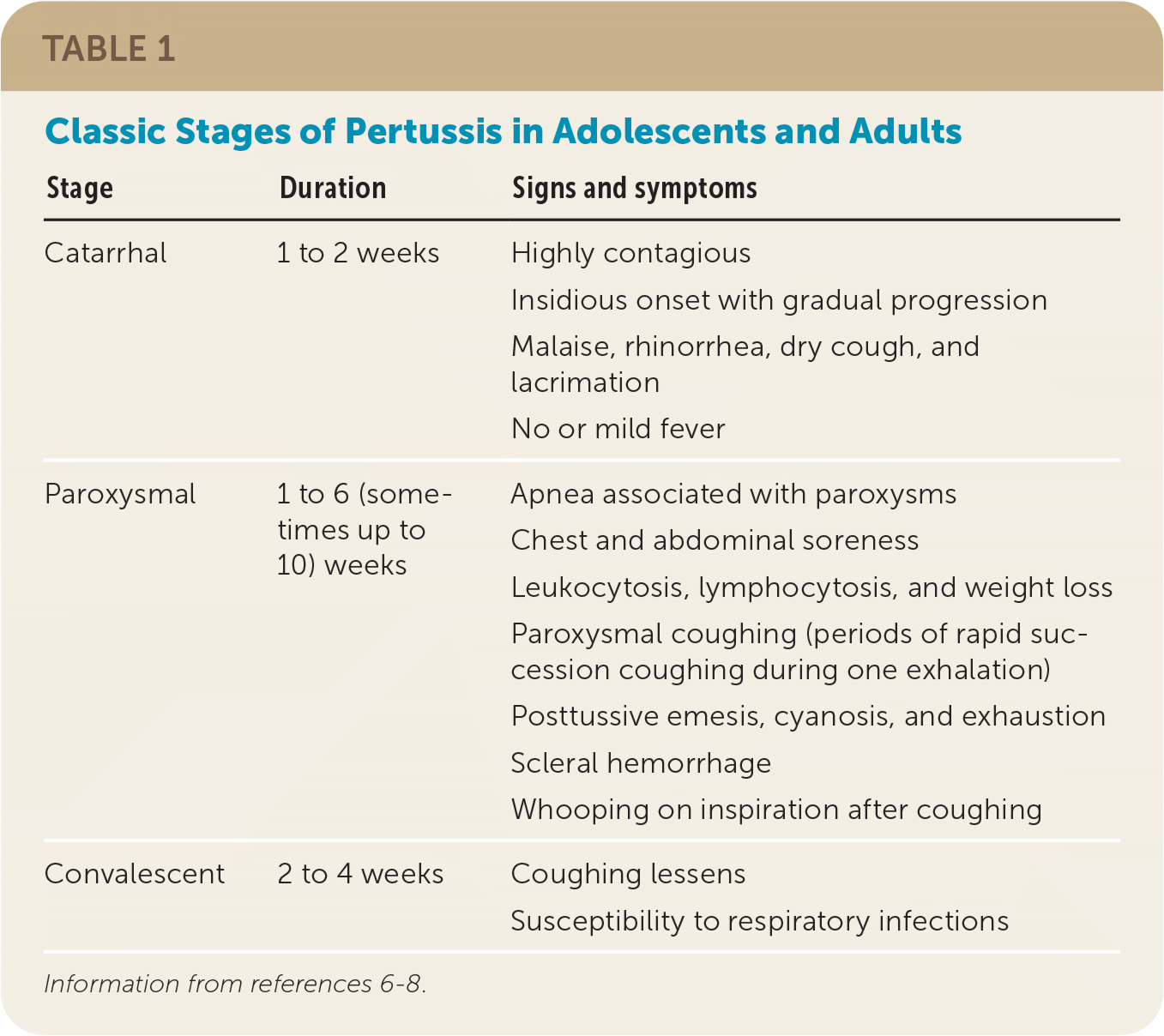
| Stage | Duration | Signs and symptoms |
|---|---|---|
| Catarrhal | 1 to 2 weeks | Highly contagious Insidious onset with gradual progression Malaise, rhinorrhea, dry cough, and lacrimation No or mild fever |
| Paroxysmal | 1 to 6 (sometimes up to 10) weeks | Apnea associated with paroxysms Chest and abdominal soreness Leukocytosis, lymphocytosis, and weight loss Paroxysmal coughing (periods of rapid succession coughing during one exhalation) Posttussive emesis, cyanosis, and exhaustion Scleral hemorrhage Whooping on inspiration after coughing |
| Convalescent | 2 to 4 weeks | Coughing lessens Susceptibility to respiratory infections |
EVIDENCE SUMMARY
The key clinical features of the initial catarrhal stage are difficult to distinguish from a viral upper respiratory tract infection and include malaise, rhinorrhea, dry cough, and lacrimation. Fever is usually mild or absent. After one to two weeks, the paroxysmal stage may manifest. Typical features of this stage include paroxysmal coughing, whooping on inspiration after coughing, and posttussive emesis.
Other common features of the paroxysmal stage include periods of apnea associated with paroxysms, chest and abdominal soreness from prolonged coughing, and scleral hemorrhage. In between paroxysms, the patient may be asymptomatic. The paroxysmal stage typically lasts up to six weeks before transitioning to the convalescent stage. In this stage, the coughing lessens but typically persists for an additional two to four weeks, with some patients experiencing symptoms for a longer period (e.g., “100-day cough”).7,10–12
Can Pertussis Be Diagnosed with Clinical Signs and Symptoms Alone, or Is Laboratory Testing Required?
The diagnosis of pertussis requires laboratory confirmation. Because early detection and treatment are critical to reducing transmission, accurate identification of the clinical features of pertussis is important to prompt testing.
EVIDENCE SUMMARY
No validated, accepted clinical decision rule has been established for diagnosing pertussis.13 However, the key clinical features of the infection are included in CDC and World Health Organization (WHO) case definitions. The CDC case definition of pertussis includes coughing of any duration and at least one sign or symptom (i.e., paroxysmal coughing, inspiratory whooping, posttussive emesis, or apnea) and contact with a laboratory-confirmed case of pertussis, or at least two weeks of coughing and one of these signs or symptoms.14 The WHO definition adds the clinical suspicion of pertussis and specifies that apnea is a criterion only in infants younger than one year.15 Although sensitive, the CDC and WHO criteria lack specificity.13
Two 2017 systematic reviews evaluated the clinical features used in the diagnosis of pertussis (Table 2).13,16 One found that the best clinical predictors of pertussis in adults were the presence of whooping (positive likelihood ratio [LR+] = 1.45) and posttussive emesis (LR+ = 1.46), and the best negative predictors were a lack of paroxysmal coughing (negative likelihood ratio [LR−] = 0.33) and presence of fever (LR− = 0.97).16 However, the second systematic review reported minimal value of individual signs and symptoms, with the clinician's overall impression having the highest likelihood ratio out of both reviews (LR+ = 3.3).13
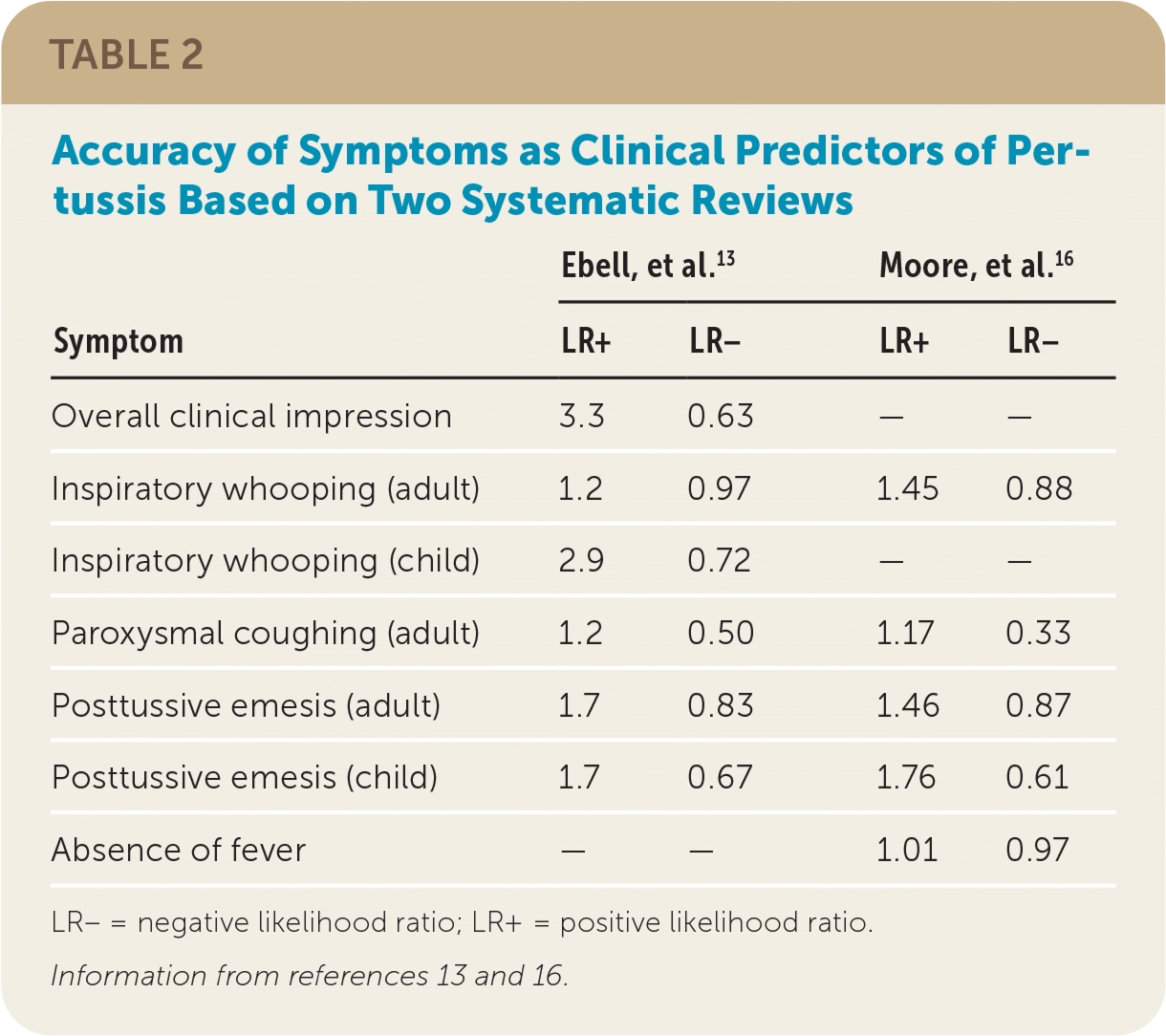
| Symptom | Ebell, et al.13 | Moore, et al.16 | ||
|---|---|---|---|---|
| LR+ | LR− | LR+ | LR− | |
| Overall clinical impression | 3.3 | 0.63 | — | — |
| Inspiratory whooping (adult) | 1.2 | 0.97 | 1.45 | 0.88 |
| Inspiratory whooping (child) | 2.9 | 0.72 | — | — |
| Paroxysmal coughing (adult) | 1.2 | 0.50 | 1.17 | 0.33 |
| Posttussive emesis (adult) | 1.7 | 0.83 | 1.46 | 0.87 |
| Posttussive emesis (child) | 1.7 | 0.67 | 1.76 | 0.61 |
| Absence of fever | — | — | 1.01 | 0.97 |
According to a 2019 guideline, pertussis should be considered likely in adults who have posttussive emesis or inspiratory whooping and unlikely if the patient has fever or lack of paroxysmal coughing.17 In children, pertussis should be considered likely if the patient has posttussive emesis, paroxysmal coughing, or inspiratory whooping.17
What Is the Preferred Diagnostic Laboratory Test for Pertussis?
Polymerase chain reaction (PCR) is the most accurate test to confirm pertussis (LR+ = 13; LR− = 0.03) and is the diagnostic test of choice in most cases.18 Culture should be used to confirm a positive PCR result during pertussis outbreaks for strain identification or if PCR is not available.7 Serology can be a helpful adjunct late in the course of illness (more than three weeks) but only in patients older than six months who have not been vaccinated in the past year.19,20
EVIDENCE SUMMARY
The sensitivities of culture and PCR are highest during the catarrhal and early paroxysmal phase, whereas the sensitivity of serology (immunoglobulin G) does not peak until late in the paroxysmal and convalescent phases (Table 3).18,19,21,22 PCR is the preferred test because it provides rapid and accurate results, whereas cultures are limited by antibiotic use and prior immunization, they are challenging to grow, and results take seven to 10 days.7
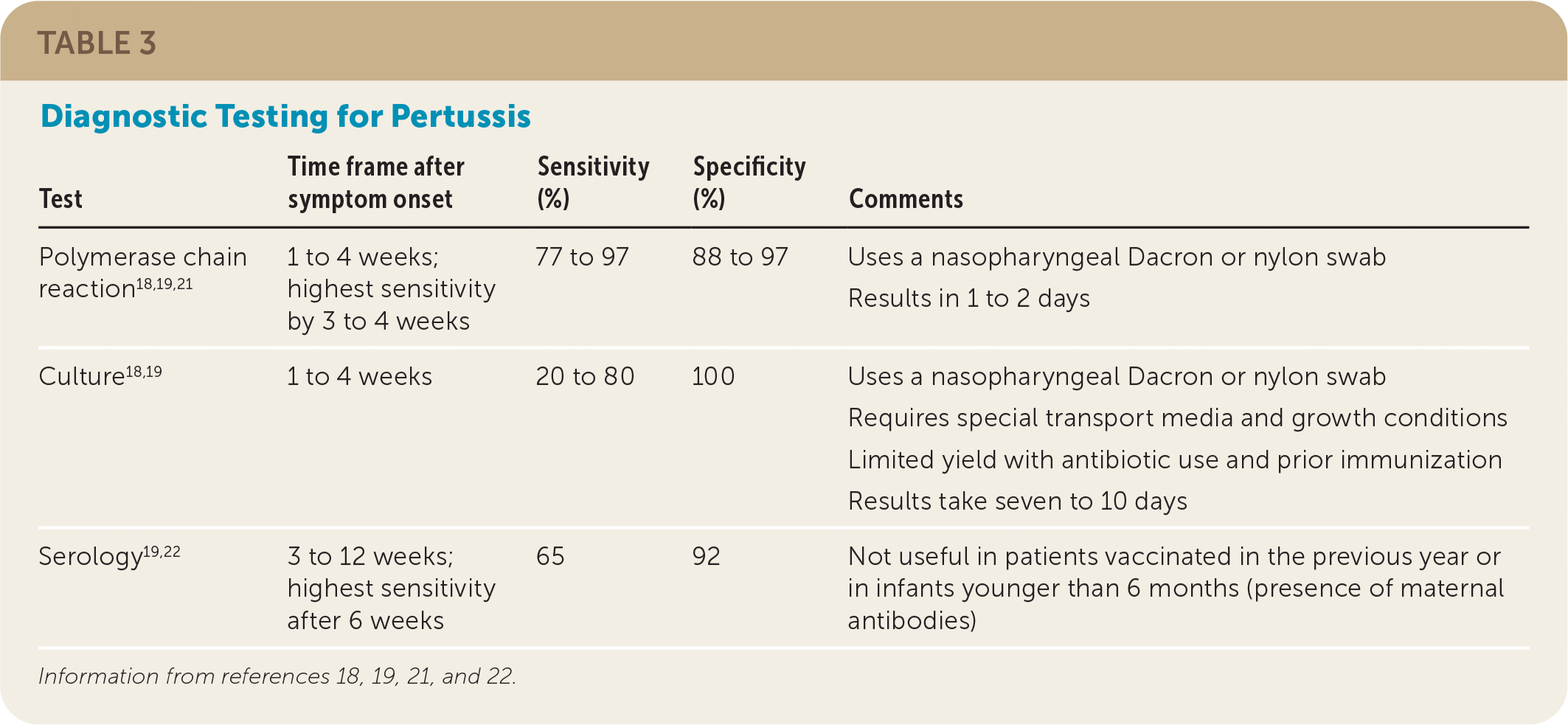
| Test | Time frame after symptom onset | Sensitivity (%) | Specificity (%) | Comments |
|---|---|---|---|---|
| Polymerase chain reaction18,19,21 | 1 to 4 weeks; highest sensitivity by 3 to 4 weeks | 77 to 97 | 88 to 97 | Uses a nasopharyngeal Dacron or nylon swab Results in 1 to 2 days |
| Culture18,19 | 1 to 4 weeks | 20 to 80 | 100 | Uses a nasopharyngeal Dacron or nylon swab Requires special transport media and growth conditions Limited yield with antibiotic use and prior immunization Results take seven to 10 days |
| Serology19,22 | 3 to 12 weeks; highest sensitivity after 6 weeks | 65 | 92 | Not useful in patients vaccinated in the previous year or in infants younger than 6 months (presence of maternal antibodies) |
Serology is primarily used for epidemiology but could be useful in patients who present late in the course of illness because it can help confirm the diagnosis as late as 12 weeks after symptom onset.6,23 Serology is not useful in patients who would be expected to have circulating antibodies (e.g., those who were recently vaccinated).24 Direct fluorescent antibody assays are no longer used because of low sensitivity and specificity.19
Why Does Pertussis Remain a Common Disease Despite High Vaccination Rates?
Some strains have shifted away from displaying the acellular vaccine antigens (i.e., pertussis toxin, pertactin, fimbriae 2 or 3, and filamentous hemagglutinin). These are known as vaccine antigen deficient strains or escape mutants.4,25,26 Even without this shift, immunity wanes in most patients because of uncertain causes.
EVIDENCE SUMMARY
Unlike with other vaccines, there is not a standardized antibody titer to confirm protection against pertussis.27 Although pertussis vaccines were initially whole cell, the high reactogenicity of the vaccine caused frequent adverse effects (e.g., local reaction, seizure, pain, fever sometimes leading to febrile seizure), resulting in the adoption of acellular vaccines in the 1990s.
Immunity typically wanes two to four years after administration of the acellular pertussis vaccine, although this can occur as early as one year postvaccination.28 Individuals with natural infection also experience waning immunity, including children with a history of pertussis.4,29 Because of this rapid decrease in immunity, it is not considered an effective public health strategy to continue recurrent tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis (Tdap) boosters.4
Why Did the Vaccine Recommendations Change for Adolescents in 2005 and Pregnant Patients in 2012?
Despite high pertussis vaccination rates in infants and children, there was a significant increase in the incidence of pertussis in the 1990s and early 2000s, especially among adolescents. Infants younger than two months have the highest risk of hospitalization and death; therefore, a passive immunity strategy (placental transmission of maternal antibodies) was adopted to protect this group.4
EVIDENCE SUMMARY
Because of the increasing incidence of pertussis in the United States and an observed waning immunity regardless of the source of inoculation (whole cell or acellular vaccine or infection), the CDC's Advisory Committee on Immunization Practices (ACIP) published updated pertussis vaccination guidelines in 2005, including introducing a booster of Tdap, rather than tetanus and diphtheria toxoids (Td), at 11 to 12 years of age or once as an adult if not previously received.4 Table 4 summarizes current pertussis vaccine guidelines.4,28
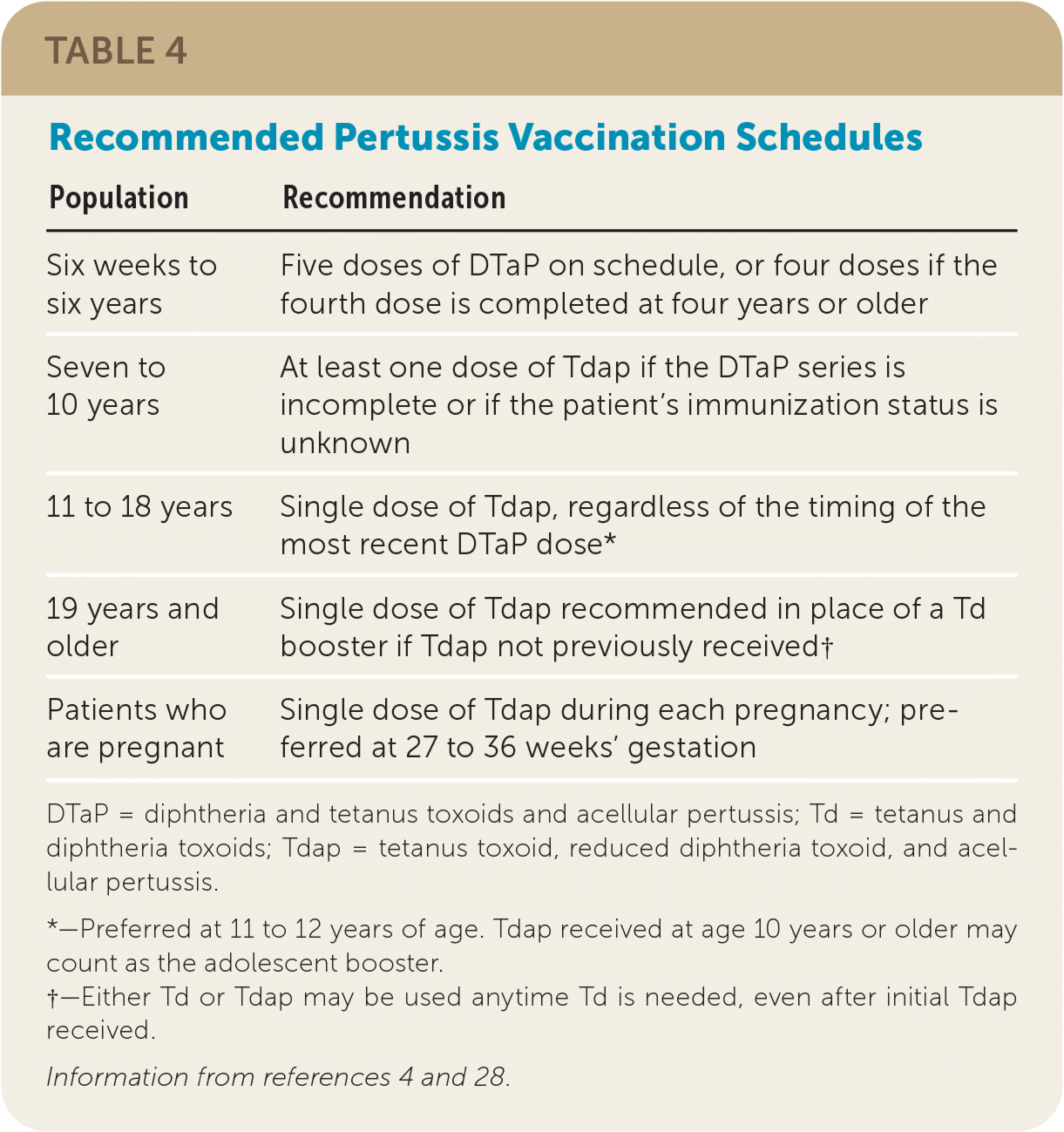
| Population | Recommendation |
|---|---|
| Six weeks to six years | Five doses of DTaP on schedule, or four doses if the fourth dose is completed at four years or older |
| Seven to 10 years | At least one dose of Tdap if the DTaP series is incomplete or if the patient's immunization status is unknown |
| 11 to 18 years | Single dose of Tdap, regardless of the timing of the most recent DTaP dose* |
| 19 years and older | Single dose of Tdap recommended in place of a Td booster if Tdap not previously received† |
| Patients who are pregnant | Single dose of Tdap during each pregnancy; preferred at 27 to 36 weeks' gestation |
Because more than 85% of pertussis deaths occur in infants too young to receive pertussis vaccination, it is recommended that pregnant patients receive the Tdap vaccine between 27 and 36 weeks' gestation with each pregnancy.4,30 This timing was recommended because of rapid waning of antipertussis antibodies and optimal timing of placental antibody transfer.30,31 If the third-trimester booster is missed, ACIP recommends not vaccinating patients with Tdap postpartum if they have ever received Tdap.4
Should Nonmaternal Caretakers of Infants or Close Contacts of Health Care Workers Be Given Tdap Boosters?
No, unless otherwise overdue for the Tdap vaccine. The target is for all individuals to receive a Tdap booster once.28 Although ACIP's 2020 recommendation allows for either Td or Tdap formulations for tetanus prophylaxis or booster, the change is essentially for the convenience of the clinician and patient because there is no identified risk of extra Tdap administrations.28
EVIDENCE SUMMARY
There is no difference in adverse effects in patients given Td or Tdap at an interval of five to 10 years, and data in pregnant patients given multiple doses in shorter time spans also have not shown an increase in adverse effects.28,32 Cocooning (vaccinating close contacts of infants and others at high risk) was based on a faulty assumption that vaccinated individuals could not transmit the disease.4 It is now known that immunized patients can contract and transmit pertussis, although the vaccine reduces disease severity in the vaccinated individual.27 Models have since shown that household siblings are more significant carriers of pertussis than the mother.4 Therefore, passive immunity through immunization of the mother during pregnancy is the primary public health goal.
If a Patient Has a Seizure After Administration of Any Dose of DTaP or Tdap, Are Additional Doses Contraindicated?
Seizure after pertussis vaccination was previously a contraindication for receiving further doses, but the risk of repeat seizure or a complicated neurologic event after subsequent diphtheria and tetanus toxoids and acellular pertussis (DTaP) or Tdap administration is low (0.5 cases of encephalopathy per 10 million doses), and further pertussis vaccination should not be avoided.4,28 Safety concerns with DTaP and Tdap are summarized in eTable A.
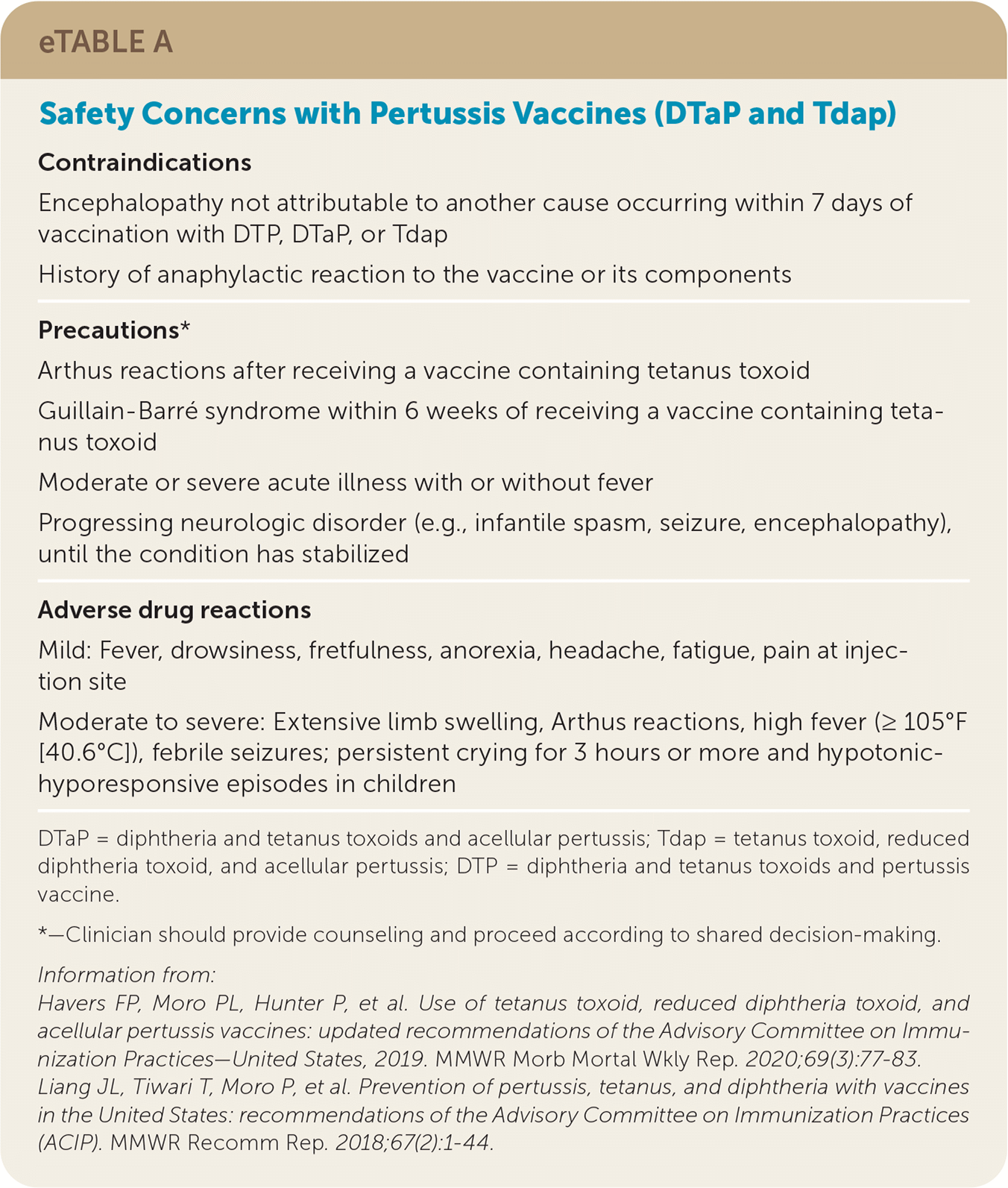
| Contraindications |
| Encephalopathy not attributable to another cause occurring within 7 days of vaccination with DTP, DTaP, or Tdap |
| History of anaphylactic reaction to the vaccine or its components |
| Precautions* |
| Arthus reactions after receiving a vaccine containing tetanus toxoid |
| Guillain-Barré syndrome within 6 weeks of receiving a vaccine containing tetanus toxoid |
| Moderate or severe acute illness with or without fever |
| Progressing neurologic disorder (e.g., infantile spasm, seizure, encephalopathy), until the condition has stabilized |
| Adverse drug reactions |
| Mild: Fever, drowsiness, fretfulness, anorexia, headache, fatigue, pain at injection site |
| Moderate to severe: Extensive limb swelling, Arthus reactions, high fever (≥105°F [40.6°C]), febrile seizures; persistent crying for 3 hours or more and hypotonic-hyporesponsive episodes in children |
EVIDENCE SUMMARY
Immunization should be temporarily delayed in a patient who has uncontrolled seizure disorder or a progressing neurologic disorder, such as infantile spasms, until the disorder is stabilized. Patients with a history of seizures that are currently controlled and patients who had a seizure with previous pertussis vaccines without prolonged symptoms of coma or encephalopathy do not have a contraindication to receiving additional doses of DTaP or Tdap vaccines.4 Children who have had persistent crying or hypotonic-hyporesponsive episodes with DTaP are unlikely to have repeat episodes with further doses; therefore, these are not contraindications to receiving DTaP or Tdap boosters.4
What Antibiotic Should Be Used to Treat Patients with Pertussis?
Antibiotics are intended to prevent transmission of pertussis to others and do not shorten the disease course or improve symptoms. Azithromycin (Zithromax) is the preferred treatment for pertussis because of its favorable safety profile, but use of other macrolides (erythromycin, clarithromycin) or trimethoprim/sulfamethoxazole is an acceptable alternative.2,33,34
EVIDENCE REVIEW
Antibiotics for pertussis have been shown to provide a microbiologic cure and are prescribed to prevent the spread of the disease within 21 days of cough onset.2,33–35 A Cochrane review did not show that antibiotics significantly reduce mortality, symptom duration, or complications in patients with pertussis.2
Azithromycin has replaced erythromycin as the preferred treatment because of its daily dosing, shorter course, and fewer gastrointestinal adverse effects.2,33,36 In adults, azithromycin can be given as a dosage of 500 mg for three days or 500 mg on day 1, then 250 mg on days 2 to 5. In children, it can be given as a dosage of 10 mg per kg for three days or 10 mg per kg on day 1, then 5 mg per kg on days 2 to 5.2,33,36
Should Pertussis-Related Cough Be Treated with Adjunct Therapies?
Treatments aimed at reducing cough, including corticosteroids, antihistamines, beta-2 agonists, and pertussis immune globulin, have not been associated with improved symptoms in patients with pertussis.37
EVIDENCE SUMMARY
A 2014 Cochrane review evaluating symptomatic treatments for pertussis, including one or two poor-quality studies per intervention, found no benefit in the reduction of coughing episodes or length of hospitalization.37 The use of symptomatic treatments is not recommended.
Who Should Receive Antibiotic Prophylaxis?
Postexposure prophylaxis should be initiated in household contacts of someone with pertussis and in those exposed to pertussis who are at high risk of severe illness or in close contact with someone who is at high risk of severe illness.38 Postexposure prophylaxis should be initiated within 21 days of exposure.38
EVIDENCE SUMMARY
The CDC recommends postexposure prophylaxis within 21 days of exposure in household contacts of someone with pertussis and in those exposed to pertussis who are at high risk of severe illness or in close contact with someone at high risk.38 Those at highest risk of severe illness include infants and people who are in their third trimester of pregnancy, are immunocompromised, or who have comorbidities that increase the risk of severe illness (e.g., respiratory disorders). The CDC has limited prophylaxis to only these groups because placebo-controlled studies of erythromycin have not demonstrated effectiveness, and a Cochrane review found no benefit of postexposure prophylaxis in those older than six months who are not in contact with an infant.2,39
This article updates previous articles on this topic by Kline, et al.,10 and Gregory.40
Data Sources: The primary resources used to identify literature were PubMed and the Cochrane database. Primary search terms included pertussis (free text) or pertussis (MeSH) and one of the following terms: treatment, complications, diagnosis, prevention, vaccination. Limits were used to identify primary literature. Essential Evidence Plus and article reference lists were reviewed to identify further sources. The CDC website was also searched using the terms above and the additional term ACIP. Search dates: April 30, 2020; June 2, 2020; July 7, 2020; October 8, 2020; and March 16, 2021.
