
This is a corrected version of the article that appeared in print.
Am Fam Physician. 2013;88(8):507-514
Patient information: A handout on this topic is available at https://familydoctor.org/online/famdocen/home/common/infections/common/bacterial/903.html.
Author disclosure: No relevant financial affiliations.
Pertussis, also known as whooping cough, is an acute respiratory tract infection that has increased in incidence in recent years. The initial catarrhal stage presents with nonspecific symptoms of malaise, rhinorrhea, sneezing, lacrimation, and mild cough. During the paroxysmal stage, severe outbreaks of coughing often lead to the classic high-pitched whooping sound patients make when gasping for breath. The paroxysmal stage is followed by the convalescent stage and resolution of symptoms. Complications vary by age, with infants more likely to experience severe complications such as apnea, pneumonia, seizures, or death. In adolescents and adults, complications are the result of chronic cough. The diagnosis depends on clinical signs and laboratory testing. Both culture and polymerase chain reaction testing can be used to confirm the diagnosis; serologic testing is not standardized or routinely recommended. Although antibiotics have not shown clear effectiveness in the treatment of pertussis, they eradicate nasal bacterial carriage and may reduce transmission rates. Macrolide antibiotics such as azithromycin are first-line treatments to prevent transmission; trimethoprim/sulfamethoxazole is an alternative in cases of allergy or intolerance to macrolides. Immunization against pertussis is essential for disease prevention. Current recommendations in the United States consist of administering five doses of the diphtheria and tetanus toxoids and acellular pertussis (DTaP) vaccine to children before seven years of age, and administering a tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis (Tdap) booster between 11 and 18 years of age. Recent efforts have focused on the vaccination of adolescents and adults, with new recommendations for a single dose of the Tdap booster if it has not been previously administered.
Most cases of pertussis are caused by Bordetella pertussis, but other species (Bordetella parapertussis and Bordetella bronchiseptica) have also been implicated.3 The reported incidence of pertussis is 8.5 cases per 100,000 persons in the overall population. In infants, the incidence is considerably higher (88.7 per 100,000).4,5 After adoption of universal vaccination in the 1940s, pertussis incidence and outbreaks declined significantly, but both have increased in recent years, prompting changes in immunization practices.
| Clinical recommendation | Evidence rating | References |
|---|---|---|
| During diagnostic testing for pertussis, a nasopharyngeal specimen should be obtained for both culture and polymerase chain reaction analysis. | C | 15, 19 |
| Pertussis should be treated with the intent of microbiological eradication and potentially decreasing transmission. Physicians should keep in mind that treatment of pertussis with antibiotics does not lead to improvement of clinical symptoms. | A | 3, 21 |
| For patients who cannot tolerate macrolide antibiotics, trimethoprim/sulfamethoxazole should be prescribed for treatment and prophylaxis of pertussis. Clindamycin is another alternative. | A | 3, 22 |
| Antibiotic prophylaxis should be considered for persons exposed to pertussis, especially those at high risk of complications, and during epidemics. | B | 3, 4, 27, 28 |
| Azithromycin (Zithromax) should be considered the preferred agent for the treatment and prophylaxis of pertussis. | A | 3, 4, 21, 22 |
| The Tdap vaccine should be administered to adolescents and adults, including those 65 years and older, in place of a single Td booster when such a booster is due. | C | 34 |
| The Tdap vaccine should be administered to children with an incomplete DTaP series when they are due for immunization, and also to persons with an unknown vaccination status. | C | 38 |
Clinical Presentation
The incubation period of pertussis is seven to 10 days, longer than the typical one- to three-day period for most viral upper respiratory tract infections. Patients then develop a presentation that can vary from asymptomatic to a mild respiratory tract infection to a persistent, severe cough. Much of this variation is due to patient age and the degree of immunity to B. pertussis.
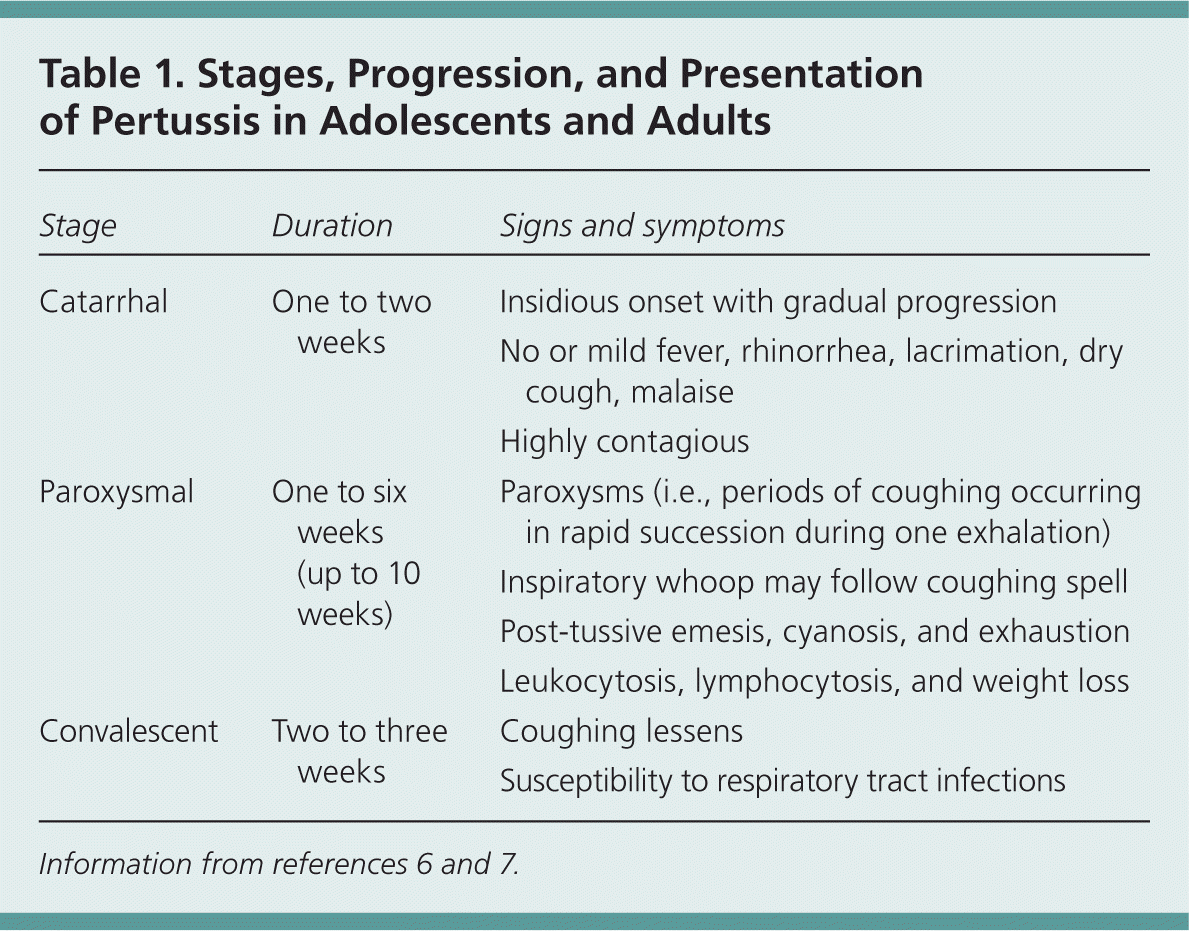
| Stage | Duration | Signs and symptoms |
|---|---|---|
| Catarrhal | One to two weeks | Insidious onset with gradual progression |
| No or mild fever, rhinorrhea, lacrimation, dry cough, malaise | ||
| Highly contagious | ||
| Paroxysmal | One to six weeks (up to 10 weeks) | Paroxysms (i.e., periods of coughing occurring in rapid succession during one exhalation) |
| Inspiratory whoop may follow coughing spell | ||
| Post-tussive emesis, cyanosis, and exhaustion | ||
| Leukocytosis, lymphocytosis, and weight loss | ||
| Convalescent | Two to three weeks | Coughing lessens |
| Susceptibility to respiratory tract infections |
Classic Presentation: Stages of Pertussis
CATARRHAL STAGE
The initial catarrhal stage lasts one to two weeks. Diagnosis during this period can be difficult, because the symptoms of pertussis are indistinct from those of routine upper respiratory tract infections.6 These symptoms include malaise, rhinorrhea, sneezing, lacrimation, and mild cough.6 Although low-grade fevers are possible, the patient's body temperature is often normal.5,6 The cough gradually becomes more frequent. During this stage, the disease is highly contagious.
PAROXYSMAL STAGE
The paroxysmal stage occurs next and is characterized by severe coughing spells. The coughing often occurs in paroxysms during a single exhalation, and is often punctuated by the classic high-pitched whoop made when the patient gasps for breath after a severe coughing attack. An audio clip of pertussis-related paroxysms is available at http://www.pkids.org/diseases/pertussis.html.
The typical paroxysmal whooping is most common in children, but may be absent in patients of any age, and is often absent in infants younger than six months, adolescents, and adults. Any infant with a severe cough, even without the classic paroxysmal whooping, may still have pertussis.
Paroxysms can last several weeks and typically occur in rapid succession because of the patient's difficulty in expelling thick mucus from the tracheobronchial tree.7 Although the paroxysms can be debilitating, patients have few other symptoms between coughing episodes. Severe paroxysms can result in post-tussive emesis, cyanosis, bulging eyes, protrusion of the tongue, salivation, lacrimation, and distention of neck veins.8 Symptoms that are unique to infants include apnea, bradycardia, and poor feeding.9
CONVALESCENT STAGE
When the severity of paroxysms wanes, the convalescent phase begins.7 This period usually lasts two to three weeks, but if the patient contracts another respiratory illness during this time, it can exacerbate the symptoms of pertussis and extend the duration of this stage.
Atypical Presentations
In adults or adolescents with acquired immunity from immunization or previous infection, symptoms of pertussis infection vary widely. When such individuals are infected with B. pertussis, they are often asymptomatic. In symptomatic persons, the illness is typically less severe and of shorter duration than that seen in children younger than four to six years. Classic pertussis can occur, but it is uncommon.8,10,11
Pertussis without classic paroxysms is the cause of up to one-third of prolonged coughs in adolescents and adults. It should be suspected as the cause of cough symptoms that persist for more than three weeks.6
Complications of Pertussis
INFANTS
Infants younger than 12 months have only partial immunity to pertussis because they have not yet completed the vaccine series. Severe complications of pertussis are thus common in this age group, especially in infants younger than six months. Complications are caused by the respiratory and systemic effects of pertussis toxin, combined with hypoxemia associated with paroxysmal cough.
Because of the high risk of morbidity and mortality in infants younger than six months, hospital admission should be considered in this age group, including access to intensive care measures. Apnea (50% of patients), pneumonia (20%), seizures (1%), and death (1%) are the most common complications in infants who are hospitalized with pertussis.12 Infants with pneumonia have a higher rate of death; they can also develop pulmonary hypertension, which can progress rapidly and be refractory to treatment.13
ADOLESCENTS AND ADULTS
In adolescents and adults, the chronic cough associated with pertussis can last up to six weeks. During that time, patients may experience weight loss (33% of patients), urinary incontinence (28%), syncope (6%), and rib fractures from severe coughing (4%).12 Other systemic complications of chronic cough include lack of sleep, subconjunctival hemorrhage, abdominal hernias, or even intracranial hemorrhage and stroke from vertebral artery dissection.14 Unlike infants, adults and adolescents with pertussis rarely develop pneumonia or require hospitalization.
Clinical Diagnosis
The clinical case definition of pertussis as specified by the Centers for Disease Control and Prevention (CDC) is a coughing illness lasting two weeks with one classic sign of pertussis (paroxysmal cough, post-tussive emesis, or inspiratory whoop), without another apparent cause.15 New case definitions that are stratified by age group have been proposed.16
Although the CDC's clinical case definition includes at least one of the aforementioned classic signs, a recent meta-analysis found that the presence or absence of classic signs in isolated cases only modestly predicted the likelihood of laboratory-confirmed pertussis.6 Therefore, physicians cannot rely solely on the clinical case definition for confirmation of infection.
The CDC states that the diagnosis of pertussis requires either symptoms that meet the case definition plus exposure to a laboratory-confirmed case of pertussis, or laboratory confirmation of infection (Table 215,17,18 ). Patients who meet the clinical definition but who cannot be linked to a confirmed case are considered to have a “probable” case of pertussis. From a clinical point of view, such individuals should be assumed to have pertussis and managed accordingly.15
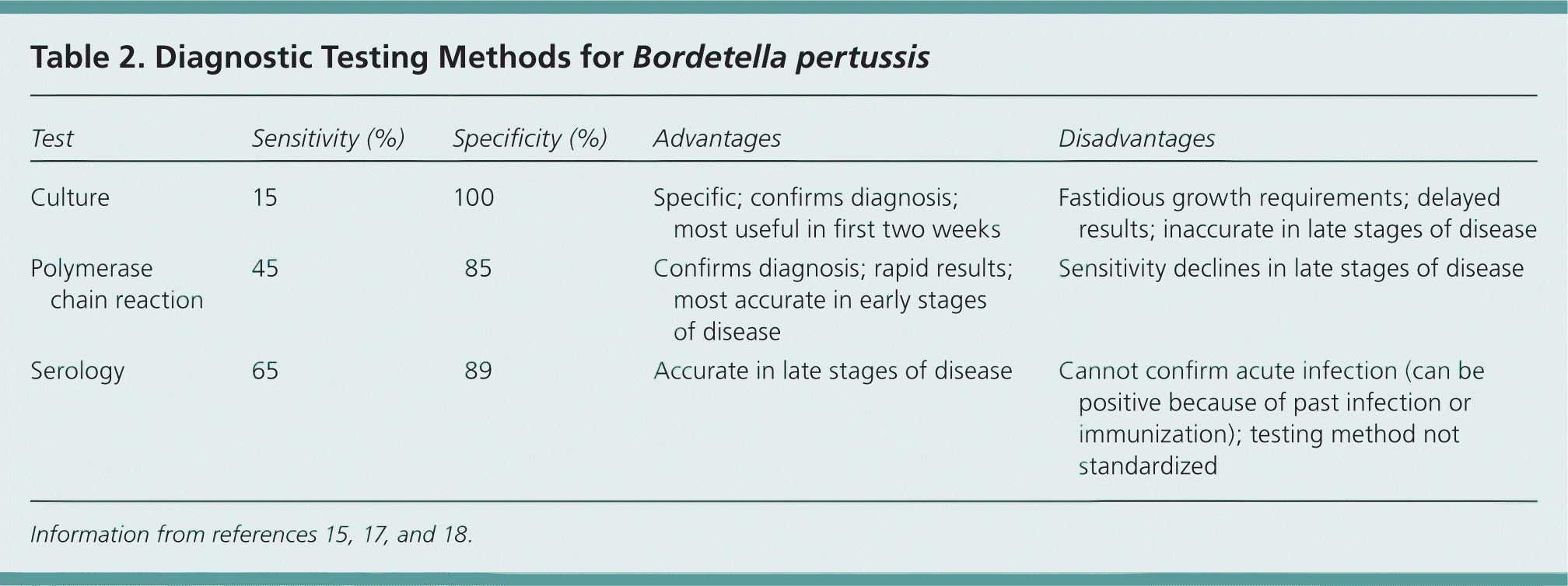
| Test | Sensitivity (%) | Specificity (%) | Advantages | Disadvantages |
|---|---|---|---|---|
| Culture | 15 | 100 | Specific; confirms diagnosis; most useful in first two weeks | Fastidious growth requirements; delayed results; inaccurate in late stages of disease |
| Polymerase chain reaction | 45 | 85 | Confirms diagnosis; rapid results; most accurate in early stages of disease | Sensitivity declines in late stages of disease |
| Serology | 65 | 89 | Accurate in late stages of disease | Cannot confirm acute infection (can be positive because of past infection or immunization); testing method not standardized |
Pertussis is a reportable disease. Both confirmed and probable cases must be reported in accordance with state laws.
Laboratory Diagnosis
The CDC recommends the use of both culture and polymerase chain reaction (PCR) testing to confirm diagnosis.15
CULTURE
Culture has long been the test of choice in the diagnosis of patients with suspected pertussis. The test is highly specific and, if positive, allows for antimicrobial sensitivity testing. However, B. pertussis is difficult to grow in culture, which results in low test sensitivity, and antibiotic use or prior immunization can further limit culture yield.
Specimens should be directly inoculated onto agar or placed into specialized transport media for delayed inoculation. Culture results may not be available for more than one week. Sensitivity is highest within the first two weeks of symptom onset.
POLYMERASE CHAIN REACTION
PCR can be used to detect DNA from pertussis and other infectious Bordetella species, and has the advantage of providing results in one to two days. Although the specificity for PCR is good (but not quite as high as culture), the sensitivity is much higher than culture.8 As a result, the use of PCR increases detection of pertussis by 19% compared with culture alone. Furthermore, antibiotic use does not affect PCR results.19
OTHER TESTS
Other methods, such as serologic testing or direct fluorescent antibody tests, are not recommended for routine use in establishing a diagnosis of pertussis.20 However, serologic testing offers the advantage of greater sensitivity for detecting infection when antibody titers are highest, which is typically two to eight weeks after the onset of symptoms.20 This method of testing could be useful in older children or adults who do not seek care until they have had a cough for many weeks.
Treatment
ANTIBIOTICS
The effectiveness of antibiotics for treating pertussis is questionable because treatment does not improve clinical symptoms.3,21 A 2007 Cochrane review concluded that antibiotics did not reduce mortality, significantly alter the course of the disease, or prevent serious complications such as pneumonia.3 Antibiotics do, however, lead to microbiological cure with eradication of the bacteria from the nasopharynx, and thus should reduce transmission of the disease to other persons.3,21,22 It is also possible that in some of the studies in the Cochrane review that showed no benefit from treatment, antibiotics were not initiated early enough to achieve significant improvement in symptoms.
Because of this possibility, and because of the potential of antibiotics to decrease transmission, the CDC continues to recommend antibiotics for the treatment of pertussis.3 Treatment should be initiated within six weeks of the onset of cough in patients younger than 12 months, and within three weeks in all other patients.3
Although erythromycin has traditionally been the antibiotic of choice, concerns over tolerability and compliance have reduced its use. Trials using erythromycin estolate, which achieves higher serum concentrations than other erythromycin salts, demonstrated fewer relapses than the other forms of erythromycin.3,23 However, this formulation is no longer available because of hepatotoxicity. Another formulation of erythromycin, delayed-release erythromycin base (Ery-Tab), is still available and included in the CDC's list of treatment options, although its use is associated with gastrointestinal effects (nausea, vomiting, and diarrhea) and hypertrophic pyloric stenosis in infants.3,22
The newer macrolide antibiotics, such as azithromycin (Zithromax) and clarithromycin (Biaxin), are better tolerated than erythromycin. They are also administered fewer times per day and have shorter treatment courses, thus making patient adherence more likely.21,24 Additionally, a meta-analysis of studies that compared azithromycin and clarithromycin with erythromycin demonstrated similar eradication rates.3 Dosing information for these medications is available in Table 3.3,4,9,21,22,24
For patients who have a macrolide intolerance, trimethoprim/sulfamethoxazole is the recommended alternative (Table 33,4,9,21,22,24 ), with clindamycin as a tertiary option.3,22 Other agents studied have been ineffective (ampicillin) or have limited effectiveness due to adverse reactions (chloramphenicol or oxytetracycline).3
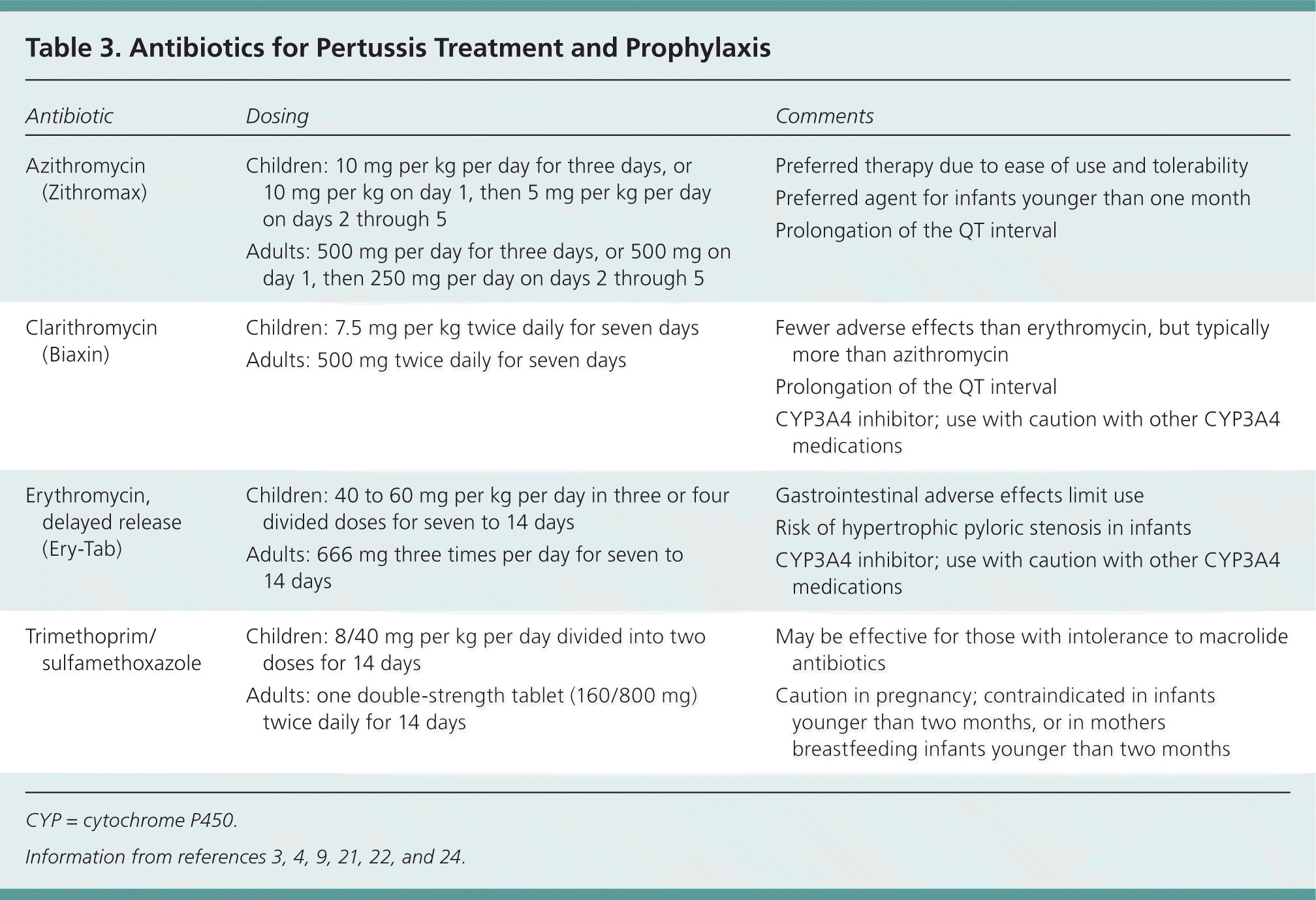
| Antibiotic | Dosing | Comments |
|---|---|---|
| Azithromycin (Zithromax) | Children: 10 mg per kg per day for three days, or 10 mg per kg on day 1, then 5 mg per kg per day on days 2 through 5 |
|
| Adults: 500 mg per day for three days, or 500 mg on day 1, then 250 mg per day on days 2 through 5 | ||
| Clarithromycin (Biaxin) | Children: 7.5 mg per kg twice daily for seven days |
|
| Adults: 500 mg twice daily for seven days | ||
| Erythromycin, delayed release (Ery-Tab) | Children: 40 to 60 mg per kg per day in three or four divided doses for seven to 14 days |
|
| Adults: 666 mg three times per day for seven to 14 days | ||
| Trimethoprim/sulfamethoxazole | Children: 8/40 mg per kg per day divided into two doses for 14 days |
|
| Adults: one double-strength tablet (160/800 mg) twice daily for 14 days |
SYMPTOMATIC TREATMENT
Treatment of pertussis symptoms using corticosteroids, bronchodilators, antitussives, and antihistamines has not been adequately evaluated. These medications are not generally recommended.25
Prophylaxis
Antibiotic prophylaxis has traditionally been used to prevent transmission to personal contacts of patients with pertussis. The CDC recommends that prophylaxis given within the first 21 days of illness be considered for all close contacts, including family members, caretakers, and health care professionals.3,4,26–28 This consideration should weigh the infectiousness of the patient (which is highest in the early stages of the illness and before resolution of symptoms in persons who have a mild form of the disease), the risk of complications for the contact if symptomatic pertussis were to develop, and the potential for the contact to spread infection to another high-risk individual (e.g., possibly spreading the infection to an infant).4,26 Persons at the highest risk of infection are infants younger than 12 months (especially those younger than six months) and women in the third trimester of pregnancy.
Despite the recommendations for treating close contacts, it should be noted that in a placebo-controlled trial of erythromycin prophylaxis, investigators found that erythromycin did not significantly reduce the frequency of either paroxysmal or whooping-type coughs in contacts, nor did it significantly reduce the number of contacts who developed a positive culture.27 Thus, a Cochrane review suggests that withholding prophylaxis can be considered for personal contacts who are older than six months if they will have no contact with infants younger than six months.3
Prevention
The prevention of pertussis centers on community vaccination. Recent immunization efforts have focused on adults, because they are the primary transmitters of the disease to infants and are at risk of infection themselves.29
Whole-cell pertussis vaccine in combination with diphtheria and tetanus toxoids was introduced in 1948.11,30 After studies raised concerns about a temporal relation between pertussis-containing vaccines and neurologic illnesses, including encephalopathy, infantile spasms, and sudden infant death syndrome, acellular pertussis vaccines were developed.11,31 Acellular pertussis vaccines include representative antigens of pathologic toxins seen in B. pertussis strains.32
CURRENT ACELLULAR VACCINES
Acellular pertussis vaccines in the United States are available in combination with diphtheria and tetanus toxoids, and include diphtheria and tetanus toxoids and acellular pertussis (DTaP) and tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis (Tdap). A key difference between the two vaccines is that DTaP has a higher concentration of diphtheria toxoid than Tdap, and has been associated with an increased risk of local reactions in patients older than six years.33 Thus, Tdap is the acellular pertussis vaccine recommended for older children, adolescents, and adults. Pertussis-only vaccines (i.e., those not combined with tetanus or diphtheria toxoids) are not available in the United States, but are available in Europe and Canada.
VACCINE RECOMMENDATIONS
Recommendations from the CDC's Advisory Committee on Immunization Practices (ACIP) pertaining to pertussis vaccination have undergone several recent revisions.34 Because of declining immunity in the years after immunization, in 2006 ACIP recommended that persons 19 to 64 years of age receive a single dose of Tdap in place of a tetanus and diphtheria toxoids (Td) booster.35,36 ACIP also recommended that children 11 through 18 years of age receive Tdap in lieu of the Td immunization previously recommended for that age group to boost immunity from the original five-dose DTaP sequence.
In 2010, ACIP expanded its recommendations to include a dose of Tdap for children seven to 10 years of age who have not received all five doses of DTaP.38 [corrected] In addition, adults 65 years and older should routinely receive Tdap.34 ACIP also suggested that Tdap be administered to all patients with unknown Td or Tdap status, and stated that there was no longer concern about a minimum time interval between administration of Td and the Tdap booster.33
In 2011 and again in 2012, ACIP revised its recommendation on Tdap for pregnant women. Women should receive a single dose of Tdap during each pregnancy, preferably at 27 to 36 weeks' gestation.37 [corrected]
In 2012, ACIP published updated childhood and adult vaccination schedules that include all of the aforementioned changes. The adult schedule includes recommendations for Tdap administration to persons with specific chronic medical problems. The current vaccination schedules are available at https://www.aafp.org/patient-care/immunizations/schedules.html.
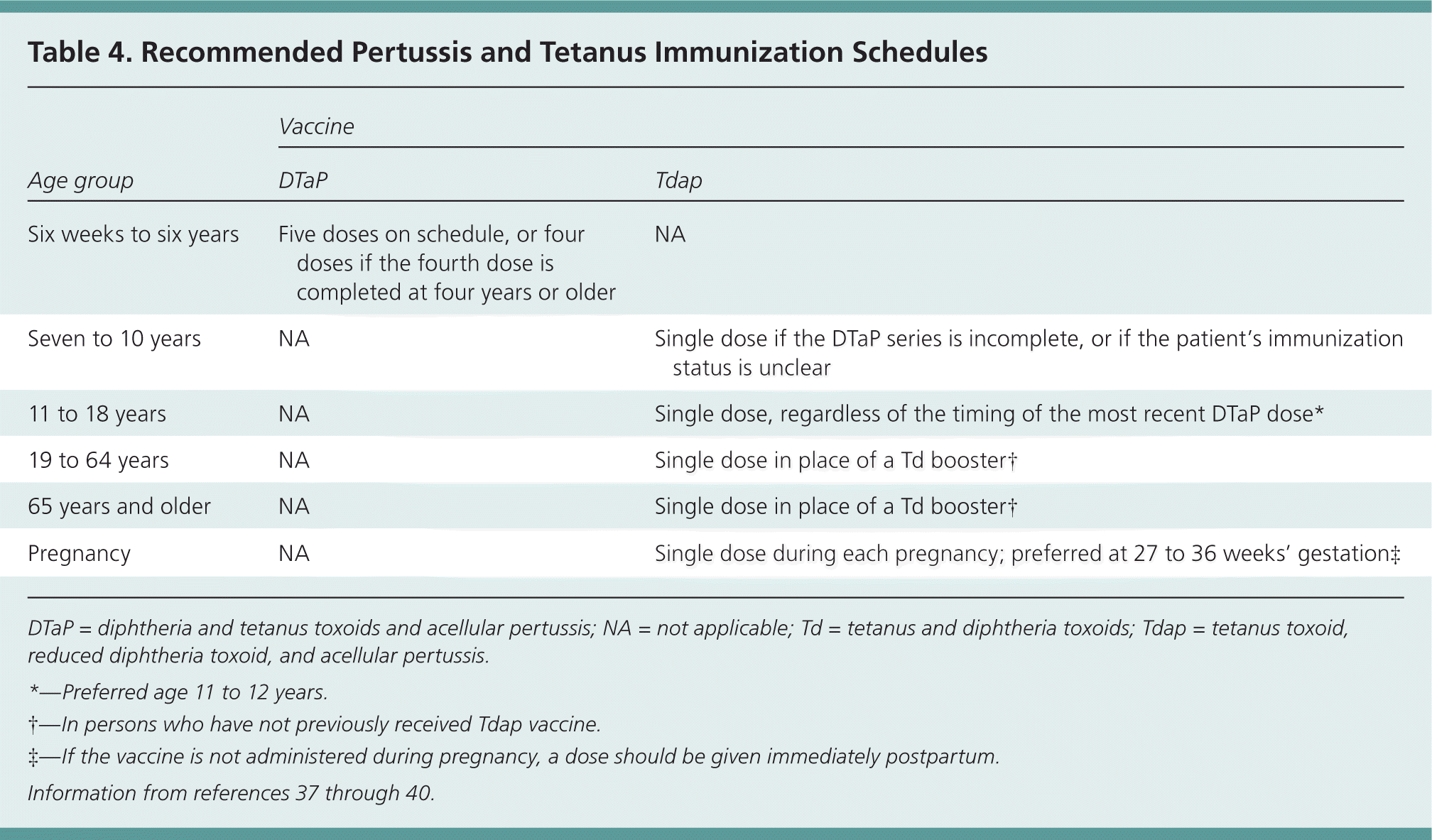
| Age group | Vaccine | |
|---|---|---|
| DTaP | Tdap | |
| Six weeks to six years | Five doses on schedule, or four doses if the fourth dose is completed at four years or older | NA |
| Seven to 10 years | NA | Single dose if the DTaP series is incomplete, or if the patient's immunization status is unclear |
| 11 to 18 years | NA | Single dose, regardless of the timing of the most recent DTaP dose* |
| 19 to 64 years | NA | Single dose in place of a Td booster† |
| 65 years and older | NA | Single dose in place of a Td booster† |
| Pregnancy | NA | Single dose during each pregnancy; preferred at 27 to 36 weeks' gestation‡ |
In 2013, a report identified pertactin-negative strains of pertussis among children hospitalized in Philadelphia for the first time in the United States.41 Although pertactin is a major component of acellular pertussis vaccines, it is unknown at this time whether this is a major factor in the waning immunity to pertussis among those previously vaccinated, or if future changes to the immunization schedule will be warranted.41
ADVERSE REACTIONS TO VACCINATION

| Contraindications |
| Encephalopathy not attributable to another cause occurring within seven days after vaccination with DTP or DTaP |
| History of anaphylactic reaction to the vaccine or its components |
| Precautions |
| Collapse or shock-like state within 48 hours of receiving DTP or DTaP vaccine |
| Guillain-Barré syndrome < six weeks after receiving a tetanus toxoid–containing vaccine |
| History of arthus (type III hypersensitivity) reactions after receiving a tetanus toxoid–containing vaccine |
| History of fever ≥ 105°F (≥ 40.5°C) within 48 hours of receiving DTP or DTaP vaccine |
| Inconsolable crying in infants/children > three hours after receiving DTP or DTaP vaccine |
| Moderate to severe acute illness, with or without fever |
| Progressing neurologic disorder (i.e., infantile spasm, seizure, encephalopathy) until condition has stabilized |
| Seizure ≤ three days after receiving DTP or DTaP vaccine |
| Adverse drug reactions |
| Mild reactions include fever, drowsiness, fretfulness, anorexia, headache, fatigue, and pain at injection site |
| Moderate to severe systemic events include extensive limb swelling, arthus reactions, temperature ≥ 105°F (≥ 40.5°C), febrile seizures, persistent crying in infants/children > three hours, and hypotonic hyporesponsive episodes |
Concern has been raised about a link between the pertussis vaccine and neurologic complications. There is a slight increase in febrile seizures occurring after the initial doses of acellular pertussis vaccine are administered in children younger than five years, but recent studies show no increase in the risk of chronic childhood seizure disorders.44–46
Data Sources: The primary resources used to identify literature were PubMed and the Cochrane database. Primary search terms included pertussis (free text) or pertussis (MeSH) and one of the following terms: treatment, complications, diagnosis, prevention, or vaccination. Limits were used to identify primary literature. Essential Evidence Plus and article reference lists were browsed to identify further sources. The CDC website was also searched with the terms above and the addition of ACIP as a search term. Search dates: January 18, 2011; February 10, 2011; November 25, 2011; April 2, 2012; and August 16, 2013.
