Am Fam Physician. 2021;104(5):509-512
Author disclosure: No relevant financial affiliations.
Key Clinical Issue
How effective are pharmacologic and nonpharmacologic acute treatments for episodic migraine in adults?
Evidence-Based Answer
Nonsteroidal anti-inflammatory drugs (NSAIDs) and triptans, individually and combined, are superior compared with placebo in resolving episodic migraine pain and are first-line choices for acute treatment. (Strength of Recommendation [SOR]: A, based on consistent, good-quality patient-oriented evidence.) Acetaminophen and dihydroergotamine also relieve migraine pain better than placebo. (SOR: A, based on consistent, good-quality patient-oriented evidence.) Calcitonin gene-related peptide antagonists and lasmiditan improve pain and function in acute migraines compared with placebo. (SOR: A, based on consistent, good-quality patient-oriented evidence.) Opioids do not improve pain or function, and adverse events are greater, compared with established migraine treatment options. (SOR: B, based on inconsistent or limited-quality patient-oriented evidence.) Acupuncture does not relieve migraine pain compared with sham acupuncture, but noninvasive vagus nerve stimulation and remote electrical neuromodulation relieve acute migraine pain compared with sham stimulation.1 (SOR: B, based on inconsistent or limited-quality patient-oriented evidence.)
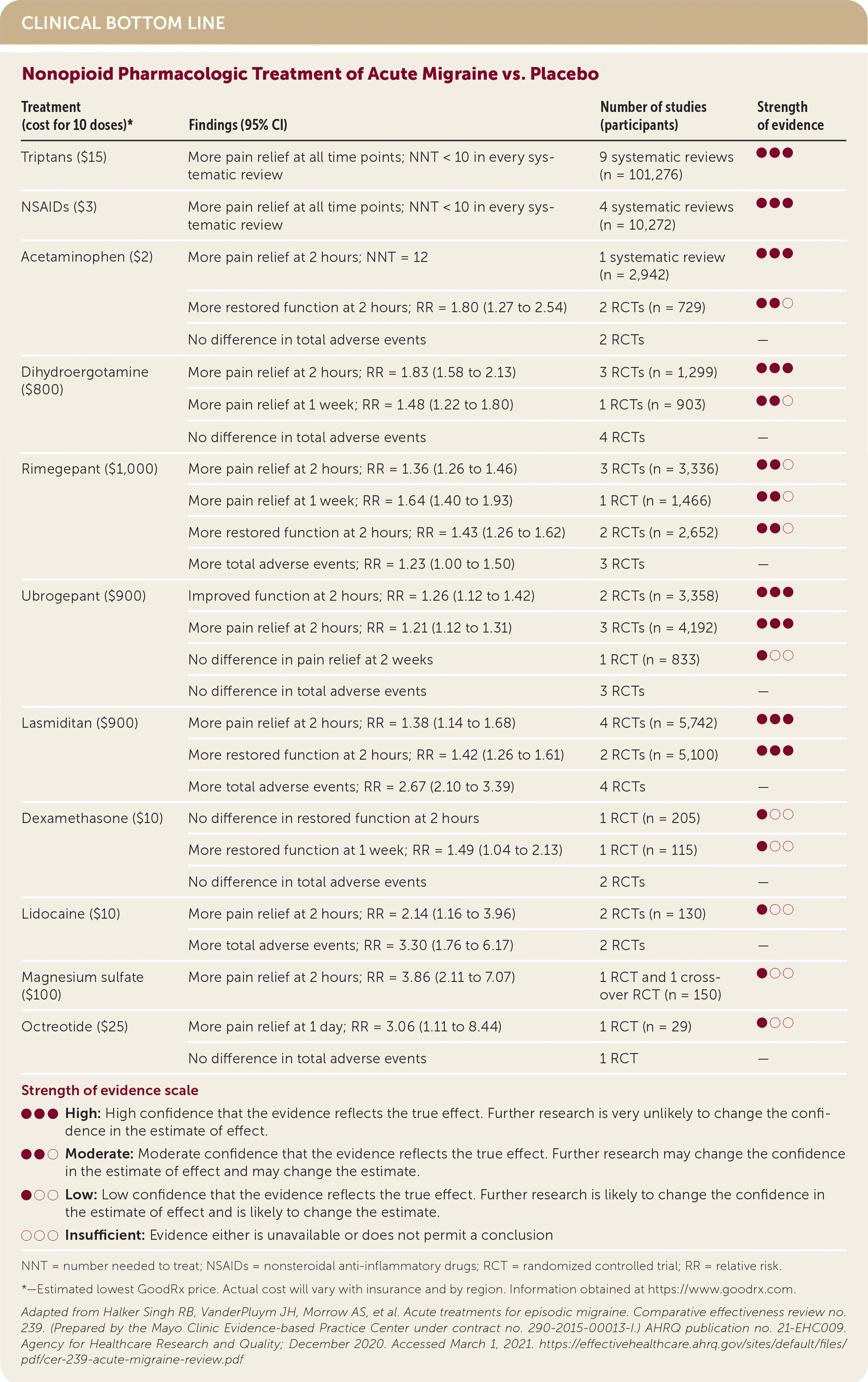
| Treatment (cost for 10 doses)* | Findings (95% CI) | Number of studies (participants) | Strength of evidence |
|---|---|---|---|
| Triptans ($15) | More pain relief at all time points; NNT < 10 in every systematic review | 9 systematic reviews (n = 101,276) | ●●● |
| NSAIDs ($3) | More pain relief at all time points; NNT < 10 in every systematic review | 4 systematic reviews (n = 10,272) | ●●● |
| Acetaminophen ($2) | More pain relief at 2 hours; NNT = 12 | 1 systematic review (n = 2,942) | ●●● |
| More restored function at 2 hours; RR = 1.80 (1.27 to 2.54) | 2 RCTs (n = 729) | ●●○ | |
| No difference in total adverse events | 2 RCTs | — | |
| Dihydroergotamine ($800) | More pain relief at 2 hours; RR = 1.83 (1.58 to 2.13) | 3 RCTs (n = 1,299) | ●●● |
| More pain relief at 1 week; RR = 1.48 (1.22 to 1.80) | 1 RCTs (n = 903) | ●●○ | |
| No difference in total adverse events | 4 RCTs | — | |
| Ergotamine plus caffeine ($90) | No difference in function | 1 RCT (n = 309) | ●○○ |
| More pain relief at 2 hours; RR = 1.61 (1.05 to 2.49) | 1 RCT (n = 309) | ●●○ | |
| No difference in total adverse events | 2 RCTs | — | |
| Chlorpromazine ($20) | No difference in function | 1 RCT (n = 36) | ●○○ |
| More pain relief at 2 hours; RR = 5.46 (2.97 to 10.05) | 2 RCTs (n = 123) | ●○○ | |
| No difference in total adverse events | 1 RCT | — | |
| Droperidol ($180) | More pain relief at 2 hours; RR = 1.39 (1.11 to 1.74) | 1 RCT (n = 305) | ●○○ |
| More total adverse events; RR = 1.61 (1.18 to 2.20) | 1 RCT | — | |
| Haloperidol ($9) | More pain relief at 2 hours; RR = 5.33 (1.84 to 15.49) | 1 RCT (n = 40) | ●○○ |
| More total adverse events; RR = 6 (2.12 to 120.65) | 1 RCT | — | |
| Metoclopramide ($3) | More pain relief at 2 hours; RR = 1.91 (1.47 to 2.48) | 3 RCTs (n = 268) | ●○○ |
| No difference in total adverse events | 2 RCTs | — | |
| Prochlorperazine ($6) | More pain relief at 2 hours; RR = 1.80 (1.10 to 2.94) | 2 RCTs (n = 90) | ●○○ |
| More total adverse events; RR = 6.48 (1.49 to 28.17) | 1 RCT | — | |
| Rimegepant ($1,000) | More pain relief at 2 hours; RR = 1.36 (1.26 to 1.46) | 3 RCTs (n = 3,336) | ●●○ |
| More pain relief at 1 week; RR = 1.64 (1.40 to 1.93) | 1 RCT (n = 1,466) | ●●○ | |
| More restored function at 2 hours; RR = 1.43 (1.26 to 1.62) | 2 RCTs (n = 2,652) | ●●○ | |
| More total adverse events; RR = 1.23 (1.00 to 1.50) | 3 RCTs | — | |
| Ubrogepant ($900) | Improved function at 2 hours; RR = 1.26 (1.12 to 1.42) | 2 RCTs (n = 3,358) | ●●● |
| More pain relief at 2 hours; RR = 1.21 (1.12 to 1.31) | 3 RCTs (n = 4,192) | ●●● | |
| No difference in pain relief at 2 weeks | 1 RCT (n = 833) | ●○○ | |
| No difference in total adverse events | 3 RCTs | — | |
| Lasmiditan ($900) | More pain relief at 2 hours; RR = 1.38 (1.14 to 1.68) | 4 RCTs (n = 5,742) | ●●● |
| More restored function at 2 hours; RR = 1.42 (1.26 to 1.61) | 2 RCTs (n = 5,100) | ●●● | |
| More total adverse events; RR = 2.67 (2.10 to 3.39) | 4 RCTs | — | |
| Dexamethasone ($10) | No difference in restored function at 2 hours | 1 RCT (n = 205) | ●○○ |
| More restored function at 1 week; RR = 1.49 (1.04 to 2.13) | 1 RCT (n = 115) | ●○○ | |
| No difference in total adverse events | 2 RCTs | — | |
| Lidocaine ($10) | More pain relief at 2 hours; RR = 2.14 (1.16 to 3.96) | 2 RCTs (n = 130) | ●○○ |
| More total adverse events; RR = 3.30 (1.76 to 6.17) | 2 RCTs | — | |
| Magnesium sulfate ($100) | More pain relief at 2 hours; RR = 3.86 (2.11 to 7.07) | 1 RCT and 1 crossover RCT (n = 150) | ●○○ |
| Octreotide ($25) | More pain relief at 1 day; RR = 3.06 (1.11 to 8.44) | 1 RCT (n = 29) | ●○○ |
| No difference in total adverse events | 1 RCT | — |
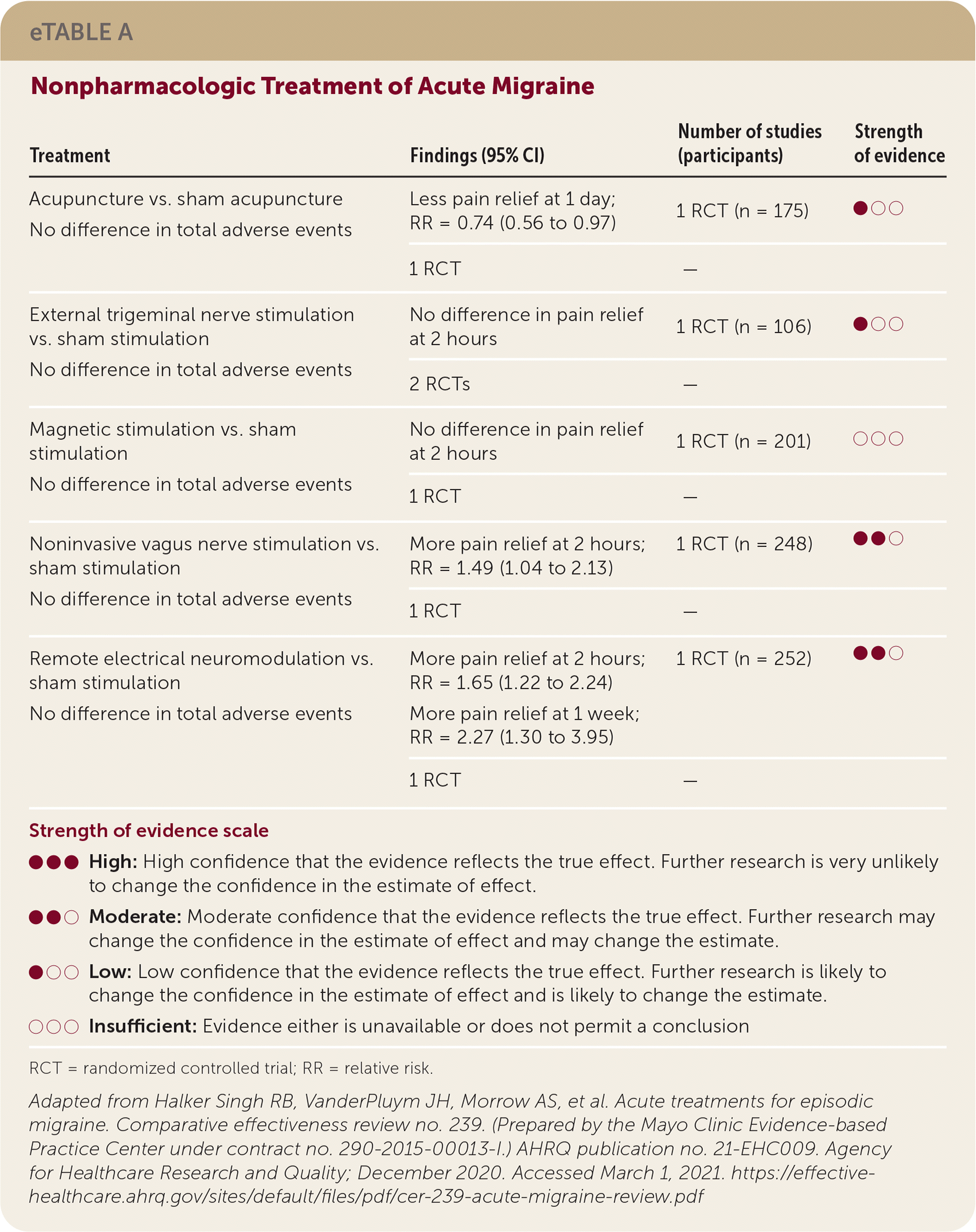
| Treatment | Findings (95% CI) | Number of studies (participants) | Strength of evidence |
|---|---|---|---|
| Acupuncture vs. sham acupuncture | Less pain relief at 1 day; RR = 0.74 (0.56 to 0.97) | 1 RCT (n = 175) | ●○○ |
| No difference in total adverse events | 1 RCT | — | |
| External trigeminal nerve stimulation vs. sham stimulation | No difference in pain relief at 2 hours | 1 RCT (n = 106) | ●○○ |
| No difference in total adverse events | 2 RCTs | — | |
| Magnetic stimulation vs. sham stimulation | No difference in pain relief at 2 hours | 1 RCT (n = 201) | ○○○ |
| No difference in total adverse events | 1 RCT | — | |
| Noninvasive vagus nerve stimulation vs. sham stimulation | More pain relief at 2 hours; RR = 1.49 (1.04 to 2.13) | 1 RCT (n = 248) | ●●○ |
| No difference in total adverse events | 1 RCT | — | |
| Remote electrical neuromodulation vs. sham stimulation | More pain relief at 2 hours; RR = 1.65 (1.22 to 2.24) | 1 RCT (n = 252) | ●●○ |
| No difference in total adverse events | More pain relief at 1 week; RR = 2.27 (1.30 to 3.95) | ||
| 1 RCT | — |
Practice Pointers
Migraine headaches are one of the most common acute medical problems.2 More than one in six adults in the United States reported having a migraine or severe headache in the past three months, and in one-half of those people, it caused severe impairment such as missing work or school.3 Episodic migraines are defined as headaches with at least two of the following: unilateral location, pulsating quality, moderate to severe pain intensity, and aggravated by or causes avoidance of routine physical activity. Episodic migraines also must be associated with nausea or photophobia and phonophobia and last four to 72 hours when untreated.4
The Agency for Healthcare Research and Quality (AHRQ) review assessed the effectiveness of pharmacologic and nonpharmacologic options for the acute treatment of episodic migraine. The review included 15 existing systematic reviews of NSAIDs and triptans and 141 studies (n = 37,653) of other migraine treatments in the outpatient or emergency department setting.1,5
The AHRQ review concludes that NSAIDs and triptans are first-line treatments of acute migraines. Four systematic reviews (n = 10,272) comparing NSAIDs with various interventions, including placebo and triptans, found that NSAIDs lead to pain relief and resolution at all time points with a number needed to treat (NNT) of less than 10 in all studies for two-hour migraine pain relief and 24-hour sustained pain relief.
Triptans improved pain and function at two and 24 hours compared with placebo in nine systematic reviews (n = 101,276) with an NNT of less than 10 in all studies. Higher doses of triptans were significantly more effective; 100 mg of sumatriptan was significantly more effective at 24-hour pain relief than a 50-mg dose (NNT = 4.5). However, an increase in doses of triptans was also associated with an increase in harms compared with placebo (e.g., sumatriptan, 100 mg [number needed to harm (NNH) = 5.2], 50 mg [NNH = 13], and 25 mg [NNH = not statistically significant]). Adverse events included nausea, dizziness, paresthesias, somnolence, and chest discomfort. Earlier treatment during the mild phase of the migraine appeared to be more effective, although none of the studies were intentionally designed to evaluate timing of administration.6
Two systematic reviews found that combining a triptan and an NSAID is effective and well tolerated, leading to relief of moderate to severe headaches at two hours compared with placebo (NNT = 3.2; NNH = 11). The most commonly reported adverse events were dizziness, nausea, dyspepsia, paresthesia, somnolence, dry mouth, and chest discomfort.1,7
The AHRQ review confirms that opioids should not be used in the acute treatment of migraines. Most of the studies that included opioids found them to be less effective and associated with more adverse events compared with other medications or placebo in the treatment of episodic migraine.1 Physicians should avoid opioids for the acute treatment of migraine in all settings if possible. This is consistent with the Choosing Wisely recommendation from the American Headache Society.8
The review supports the guidance that physicians should use NSAIDs for acute treatment of mild or moderate migraines and triptans for treatment of moderate or severe migraines.4,9 One important exception to this guidance is pregnancy, during which acetaminophen is the preferred pharmacologic choice.10 In cases where NSAIDs or triptans are not effective or only partially effective, a trial of combination therapy would be appropriate. Because many studies included in the systematic reviews of NSAIDs and triptans compared antiemetics in combination with NSAIDs or triptans, using a combination of NSAIDs and/or triptans with antiemetics would be reasonable. If NSAIDs and triptans are ineffective or contraindicated, there are multiple options with moderate- to high-quality evidence of effectiveness including dihydroergotamine, antiemetics, acetaminophen, rimegepant, ubrogepant, and lasmiditan. Physicians should consider individual patient factors and access to medication when evaluating treatment choices after NSAIDs and triptans.
Physicians could try noninvasive vagus nerve stimulation (a hand-held device placed on the side of the neck that applies low-voltage electrical pulses) and remote electrical neuromodulation (a wearable device placed on the upper arm that applies low-voltage electrical stimulation) if they are available. These may be effective in acute migraine treatment, although this is based on a single randomized controlled trial for each device. A noninvasive vagus nerve stimulation device for the treatment of episodic cluster headaches and acute migraines and a remote electrical neuromodulation device for the treatment of acute migraines have been classified as de novo devices by the U.S. Food and Drug Administration. Both devices require a prescription. Adverse events reported include dermatologic reactions such as redness, pain, and warmth.11–13 The effectiveness and safety of these nonpharmacologic devices have not been as well studied as pharmacologic acute migraine treatments.
The review focused on the treatment of episodic migraine headaches. Migraine prevention medications should be considered in patients with more than three migraine headache days a month, who require more than 10 days of acute treatments each month, who experience adverse effects from acute treatments, or for whom migraine headaches significantly interfere with their daily life.4
Editor's Note: American Family Physician SOR ratings are different from the AHRQ strength of evidence ratings.