This is an updated version of the article that appeared in print. Updated: November 4, 2021
Am Fam Physician. 2021;104(5):500-508
Patient information: See related handout on breast implants, written by the authors of this article.
Author disclosure: No relevant financial affiliations.
Breast implants are used for a wide range of cosmetic and reconstructive purposes. In addition to breast augmentation, implants can be used for postmastectomy breast reconstruction, correction of congenital breast anomalies, breast or chest wall deformities, and male-to-female top surgery. Breast implants may confer significant benefits to patients, but several factors are important to consider preoperatively, including the impact on mammography, future lactation, and potential long-term implant complications (e.g., infection, capsular contracture, rupture, and the need for revision, replacement, or removal). A fundamental understanding of implant monitoring is also paramount to implant use. Patients with silicone breast implants should undergo routine screening for implant rupture with magnetic resonance imaging or ultrasonography completed five to six years postoperatively and then every two to three years thereafter. With the exception of complications, there are no formal recommendations regarding the timing of breast implant removal or exchange. Women with unilateral breast swelling should be evaluated with ultrasonography for an effusion that might indicate breast implant–associated anaplastic large cell lymphoma. There are no specific breast cancer screening recommendations for patients with breast implants, but special mammographic views are indicated to enhance accuracy. Although these discussions are a routine component of consultation and postoperative follow-up for plastic surgeons performing these procedures, family physicians should have a working knowledge of implant indications, characteristics, and complications to better counsel their patients, to ensure appropriate screening, and to coordinate care after surgery.
Breast implants are used for cosmetic and reconstructive purposes. Implant placement for primary breast augmentation is the most common cosmetic surgical procedure in the United States, with more than 313,000 procedures performed in 2018.1 Breast implants also play an important role in reconstructive procedures for breast hypoplasia,2 congenital breast anomalies,3 male-to-female top surgery,4 and postmastectomy breast reconstruction. Rates of breast reconstruction after mastectomy have increased since the passage of the Women's Health and Cancer Rights Act in 1998, which mandates insurance coverage for all stages of postmastectomy reconstruction.5 It also includes coverage of symmetry procedures for the contralateral breast in the case of a unilateral mastectomy.5 Implant-based breast reconstruction is more common than tissue-based (autologous) reconstruction, which commonly uses abdominal tissue for breast reconstruction, for patients who have undergone mastectomy.6 Operative techniques for breast implant placement can have important implications when assessing and examining patients. Notably, implants can be placed above the pectoralis major muscle, where they are more easily palpable, or below the pectoralis major muscle, where features such as implant rupture may be more difficult to discern on examination. Postmastectomy reconstruction improves patient-reported outcomes in psychosocial well-being, sexual well-being, and overall chest satisfaction.7
WHAT'S NEW ON THIS TOPIC
Breast Implants
In September 2020, the U.S. Food and Drug Administration released new guidance about labeling of breast implants to improve risk communication:
A boxed warning denotes risks such as breast implant–associated anaplastic large cell lymphoma and potential need for additional operations
A patient decision checklist should be provided to document discussion of alternatives to breast implants, risks of breast implant surgery, breast implant–associated anaplastic large cell lymphoma, systemic symptoms, and considerations for a successful breast implant candidate
Chemical materials contained in implants should be described
Silicone rupture screening guidelines
All patients should be given an implant device card
What Are the Key Characteristics of Breast Implants?
Several variables relating to the breast implant and operative technique can affect the outcome of a cosmetic or reconstructive procedure. These variables (Table 1) include the location of the operative incision, implant fill type (silicone vs. saline), and surface texture (smooth vs. textured).

| Implant variables |
| Fill type |
| Silicone: commonly chosen by patients because of its more natural feel; it carries risk of silicone leakage into breast parenchyma with rupture |
| Saline: less natural feel; saline is safely absorbed in cases of rupture |
| Size |
| Implants range, on average, from 150 to 800 mL |
| Outer shell texture |
| Smooth: slightly higher rates of capsular contracture |
| Textured: lower rates of capsular contracture, although currently not in use because of association with breast implant–associated anaplastic large cell lymphoma |
| Procedure variables |
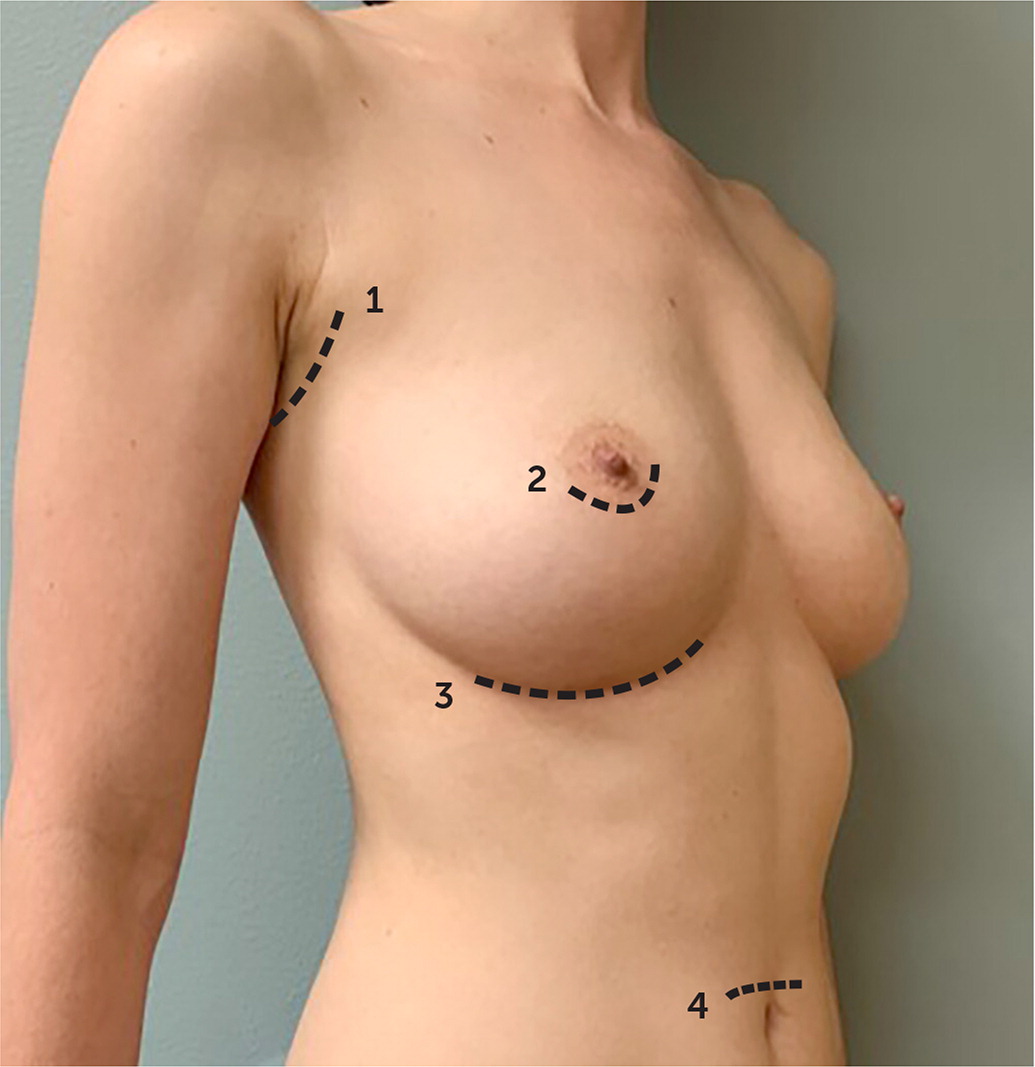
| Incision placement (Figure 1) |
| Transaxillary |
| Periareolar |
| Inframammary |
| Transumbilical |
| Implant pocket |
| Prepectoral (above pectoralis major muscle): more common in augmentation, reduces postoperative pain, avoids implant displacement with contraction of pectoralis muscle (animation deformity) |
| Submuscular (below pectoralis major muscle): more common in reconstruction, provides better implant coverage after mastectomy (reduces infection, implant exposure), reduces visible implant rippling, carries risk of animation deformity |
EVIDENCE SUMMARY
Decisions regarding implant characteristics are based on patient preference and surgeon experience. Operative decisions, including incision type and whether the implant is placed above (prepectoral pocket) or below the pectoralis muscle (submuscular pocket; Figure 1), are dependent on the indication for the procedure, anatomy, surgeon, and patient preference. A recent meta-analysis demonstrated that for breast augmentation, the periareolar approach—although cosmetically favorable—is associated with higher rates of capsular contracture, defined as thickening and hardening of scar tissue around the implant, than transaxillary or inframammary incisions.8
Silicone implants are more commonly used than saline in augmentation and postmastectomy reconstruction.9 Implants with a textured outer shell (referred to as textured implants) became popular secondary to reduced rates of capsular contracture compared with those with a smooth outer shell; however, they are currently not in use because of association with breast implant–associated anaplastic large cell lymphoma (ALCL).10
What Are the Acute Complications Associated with Breast Implants?
Most acute complications following breast augmentation or implant-based reconstruction are managed immediately by the surgical team (e.g., hematoma), but some may arise outside of the immediate perioperative period and present to the family physician. The most acute and time-sensitive complications include hematomas and an implant or tissue expander infection.
EVIDENCE SUMMARY
Rates of acute infection range from 1% to 2.5% for cosmetic procedures.11 Risk factors for infection in patients with breast implants include obesity, diabetes mellitus, smoking, mastectomy skin-flap necrosis, lymph node dissection, and radiation therapy.12 Acute infections generally present within the first four weeks after breast implant or tissue expander placement with unilateral breast pain, redness, swelling, and warmth. Constitutional symptoms may also be present. Infection severity can range from superficial cellulitis to abscess formation and sepsis. The most common source of infection is from gram-positive bacteria.11 Management of superficial infections may be initiated by the primary care physician with oral antibiotics; the plastic surgeon should be contacted for discussion and follow-up evaluation. More severe infection warrants admission for intravenous antibiotics and, in some cases, surgical washout with removal of the expander or implant. Prompt referral to the operating surgeon for management of antibiotics and possible implant removal is recommended for patients. The rate of implant salvage for infected breast implants used for reconstructive purposes is approximately 58%,13 and for augmentation it approaches 90% with medical management and/or washout in the operating room.14,15
Other complications in the postoperative period include hematoma or seroma formation and wound healing complications. These may require operative exploration, percutaneous drainage, or ongoing wound care. If diagnosed in the primary care physician's office, these patients warrant prompt referral to the surgical team for further management.
What Are the Chronic Complications of Breast Implants?
In the years following breast implant placement, patients may experience chronic complications including, but not limited to, capsular contracture and implant rupture.
EVIDENCE SUMMARY
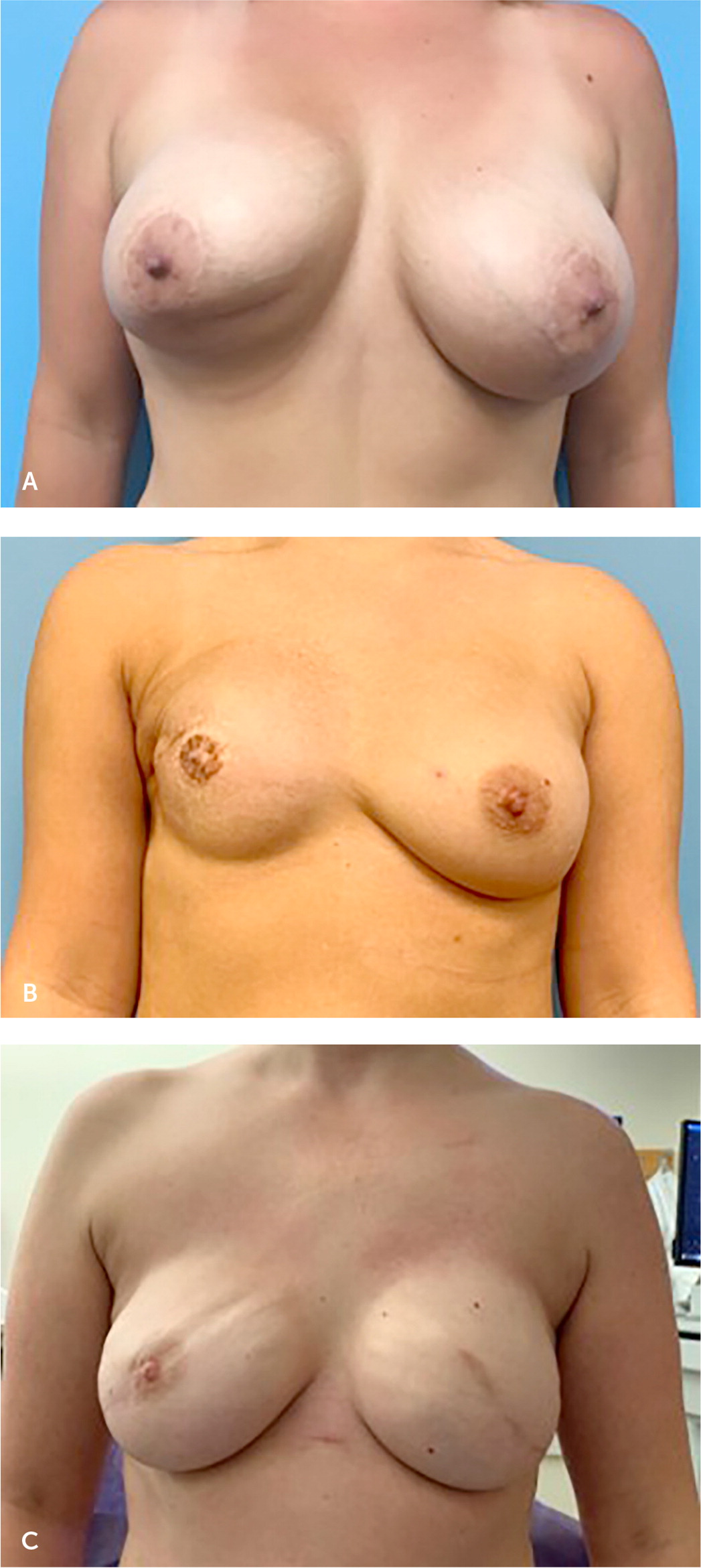
Capsular contracture is the most commonly reported complication following breast implant surgery and refers to thickening and calcification of scar tissue around the implant16 (Figure 2A). The implant becomes more palpable, visible, and, in severe cases, distorted and painful. Ten-year follow-up data for silicone implants reported capsular contracture rates of 18.9% and 24.6% for primary augmentation and reconstruction, respectively,17 whereas 10-year data for saline implants reported capsular contracture rates as high as 20.8% for augmentation and 51.7% for reconstruction.18 A capsule forms around each breast implant and over time becomes more prominent in some patients. In mild cases, this may manifest as a palpable capsule, but in more severe cases it can progress to become painful as a result of breast distortion. Although a palpable capsule is generally not an indication for surgical intervention, pain or breast distortion associated with a firm capsule may require referral for operative intervention.
Implant rupture occurs when a loss of integrity of the outer shell occurs, causing the contents to leak into the surrounding tissue. Ruptured implants necessitate a surgical referral for removal or replacement, but implant removal is not emergent and can take place on an elective basis. When saline implants rupture, the fluid is absorbed by the body, and the implant collapses with notable change in the size of the breast. Silicone implants, in contrast, typically maintain their shape after rupture because of inherent cohesivity of the gel. Silicone slowly extravagates into the surrounding capsule (intracapsular rupture) and, in more severe cases, beyond the capsule and into the breast tissue (extracapsular rupture). Silicone that invades the breast tissue can be problematic because of subsequent interference with mammography, uptake into regional lymph nodes, and the formation of silicone granulomas that may present as breast nodules.19 The U.S. Food and Drug Administration (FDA) recommends that patients with silicone breast implants undergo routine screening for implant rupture with magnetic resonance imaging (MRI) or ultrasonography five to six years postoperatively and then every two to three years thereafter.20 Importantly, the recommendations for MRI are meant to proactively identify implant rupture. There is no role for MRI in the detection or secondary prevention of breast implant–associated ALCL.
The average lifespan of a breast implant varies. A single surgeon's series of 539 breast implant removal procedures reported the time to explantation was 7.5 years for saline implants and 4.9 years for smooth silicone implants.21 Reasons for explantation varied; approximately one-half of saline and smooth gel implants were removed because of implant performance failures such as rupture, capsular contracture, pain, malrotation, and rippling. For implants removed secondary to rupture, the mean time to rupture was 8.4 years and 8.1 years for saline and smooth gel implants, respectively.21 The FDA guidance states that a breast implant is not a lifelong device and that the implant may rupture or leak at any time.22 There are no formal recommendations regarding the timing of breast implant removal or exchange, and many patients keep their implants in place until they experience a complication or implant rupture.
Other complications that may arise and necessitate operative revision include implant asymmetry or malposition (Figure 2B) or implant rippling (Figure 2C). An animation deformity, or visible implant malposition with contraction of the pectoralis muscle, is another common complication of breast implants. Animation deformity occurs when the implant is placed in the submuscular pocket. Contraction of the pectoralis muscle causes a superolateral displacement of the implant, which causes distortion of the breast skin and nipple. This can be bothersome to patients and may be managed surgically with denervation of the pectoralis muscle or transposition of the implant into the prepectoral pocket. Although none of these pose an immediate health risk, they warrant a referral to a plastic surgeon for further evaluation.
What Are Important Considerations Regarding Breast Implant–Associated ALCL?
Breast implant–associated ALCL is a rare subtype of non-Hodgkin lymphoma associated with textured breast implants. This entity was first described in 1997 in a woman with a breast mass.23
EVIDENCE SUMMARY
As of January 2020, the FDA has received reports of 733 cases of breast implant–associated ALCL.23 The incidence is estimated at one in 30,000 patients with breast implants but may be higher.24,25 A strong association occurs between ALCL and textured breast implants, although the mechanism is unknown. Breast implant–associated ALCL occurring in patients with smooth surface implants appears to be far less common, with a cumulative combined global and U.S. total of 28 in 2020.10,23,26 Saline and silicone fill implants have been associated with breast implant–associated ALCL.23
Breast implant–associated ALCL is initially diagnosed based on clinical symptoms and/or physical examination. Patients most commonly present with a peri-implant effusion27 between eight and 10.9 years after implant placement.24,28,29 A painless unilateral breast enlargement with a fluid collection may be palpable on physical examination. A minority of patients have presented with a breast mass with or without an effusion; in rare cases, patients had neither an effusion nor a mass but presented with erythematous cutaneous nodules30 or B-type symptoms such as fever, drenching night sweats, and greater than 10% weight loss over six months.31 Axillary lymphadenopathy was palpable in 15% to 34% of patients.28,29 There are no published reports of cases diagnosed on routine mammography or MRI.
Ultrasonography is the most sensitive means of detecting effusions (84%) and is generally the preferred study to evaluate a woman with unilateral breast swelling.32 MRI is also used and has an 82% sensitivity for detecting effusions.32 Mammography is limited in its ability to detect effusions or chest wall masses. Fine-needle aspiration is diagnostic and is performed by a surgical team. The workup of the fluid aspirate includes CD30 immunohistochemistry, cell morphology, and flow cytometry for T-cell characterization. These patients are treated by a multidisciplinary team including surgical oncology, medical oncology, plastic surgery, breast radiology, and hematopathology.
Breast implant–associated ALCL is indolent and generally remains confined to the breast, with an excellent prognosis. Five-year overall survival is more than 90%, and optimal treatment normally includes surgical excision (with implant removal and total capsulectomy) and chemotherapy.28
The discovery of breast implant–associated ALCL prompted Allergan, Inc., to voluntarily recall all textured implants and tissue expanders in 2019.33 The FDA does not currently recommend the removal of textured tissue expanders or implants or screening in asymptomatic patients34; however, the FDA has recommended a boxed warning for implants because of this association outlining risks including the risk of death.35 The FDA now also limits the sale and distribution of breast implants to entities that utilize a checklist to standardize communication with patients about implant complications and risks. [Updated, November 4, 2021]
What Are Breast Cancer Screening Considerations for Patients with Breast Implants?
EVIDENCE SUMMARY
In addition to routine mammography views, additional displacement views described by Eklund in the 1980s remain the standard of care for breast implants.38 Despite these additional views, obscuration of breast tissue caused by the radiopaque implant may decrease mammographic sensitivity because breast tissue closer to the chest wall is more difficult to visualize with a breast implant in place.39
Importantly, routine screening mammography is recommended based on age and personal risk factors for patients, despite the presence of an implant. MRIs for implant rupture surveillance do not replace mammographic screening.
What Impact Do Breast Implants Have on Breastfeeding?
Breastfeeding following implant placement is often possible; however, augmentation can sometimes negatively affect a mother's ability to do so.
EVIDENCE SUMMARY
The degree to which augmentation impacts the ability to breastfeed remains controversial. One study reported a significant decrease in breastfeeding of a second child after augmentation in comparison with the first birth without augmentation.41 A recent meta-analysis of cohort and cross-sectional studies demonstrated that women with breast implants were significantly less likely to breastfeed at all compared with those without implants.39 Additionally, there was a significant decrease in exclusive breastfeeding in a study of participants with breast implants compared with those without.42
A multicenter retrospective study of French university hospitals found that 75% of patients who became pregnant following breast augmentation and wanted to breastfeed were able to do so to some extent.43 The ability to breastfeed does not appear to be affected by implant size nor surgical incision approach.42,43
Historically, several reasons for decreased milk production in patients with breast implants have been suggested, including damage to the intercostal nerves supplying the breast, interruption of or pressure on milk ducts and glandular damage as a result of parenchymal dissection and implant placement, or risk of scar tissue burden.44 However, the degree to which some or all of these contribute is not well understood.
These findings underscore the importance of preoperative counseling for those considering breast augmentation and breastfeeding support and close follow-up after delivery for patients who have breast implants at the time of pregnancy.
Is Preprocedural Antibiotic Prophylaxis Necessary for Patients with Breast Implants?
Prosthetic device infection via hematogenous spread may be a concern among patients with breast implants. However, the evidence for preprocedural antibiotic prophylaxis for patients with breast implants is limited.
EVIDENCE SUMMARY
A prospective multicenter cohort study found that for patients undergoing implant-based reconstruction after mastectomy, infections may present late (30 days to one year postoperatively).45 Radiation therapy and increased body mass index were identified as risk factors for late surgical-site infection in this group.
Late onset implant seeding with infection (as opposed to an acute infection in the postoperative period) may be attributable to hematogenous spread to the capsule and implant from a distant infectious site or after an invasive procedure. There are case reports of implant infections following extensive dental work as well as a chronic foot ulcer as a source of hematogenous spread.46,47 However, review of the literature revealed no reports of late onset implant infection following routine colonoscopy, transesophageal echocardiography, or upper endoscopy. The Canadian Dental Association recommends consideration of antibiotic prophylaxis before invasive dental treatment only for patients who had implant complications (namely infection) postoperatively, but even in this circumstance it leaves the decision to the discretion of the surgeon and patient.48 There are no recommendations for antibiotic prophylaxis for routine procedures.
For patients who develop sepsis or bacteremia from other causes, implants may be seeded. Subsequent breast tenderness, erythema, or warmth should be evaluated expeditiously to reduce the ultimate risk of explantation.
What Are the Symptoms of Breast Implant Illness?
The question of breast implants' possible role in systemic or autoimmune disease has been a longstanding topic of discussion. Patients present with self-reported symptoms that may include brain fog, joint pain, rashes, fatigue, and hair loss. They attribute these symptoms to their breast implants (either silicone or saline) and generally request implant removal with total capsulectomy.49 There is no good evidence, however, that demonstrates symptom resolution with removal of implants.
EVIDENCE SUMMARY
Diagnosis of breast implant illness is complicated by the lack of consistent symptoms or diagnostic testing. Several theories about the rise of breast implant illness have been proposed and range from activation of the innate and adaptive immune pathways to a possible role of increased reporting on social media.53,54 Although the cause and true prevalence of breast implant illness remains a topic of continued research, patients with such symptoms are encouraged to report them to the FDA. A recent study suggests that a subset of women will be susceptible to autoimmune dysautonomia due to stimulation from silicone.55 Those desiring implant removal warrant referral to a plastic surgeon.56
What Are Indications for Implant Removal?
Breast implants are not permanent devices, and several factors may warrant implant removal.
EVIDENCE SUMMARY
Indications for breast implant removal include implant rupture, patient preference, including in the context of breast implant illness, capsular contracture, or breast implant–associated ALCL. At the time of implant removal, techniques for capsule management may include a simple capsular incision (capsulotomy), partial or total capsulectomy, or excision of the capsule with a margin of healthy tissue (en bloc resection). Although the former techniques are largely dependent on the reason for implant removal and the condition of the capsule, en bloc resection is indicated only for patients with breast implant–associated ALCL.57
Data Sources: A PubMed search was completed in Clinical Queries using the key terms breast implants, breast implant illness, and breast implant–associated large cell anaplastic lymphoma. The search included meta-analyses, randomized controlled trials, clinical trials, and reviews. We also searched the Agency for Healthcare Research and Quality Evidence reports, Clinical Evidence, the Cochrane database, Essential Evidence Plus, the Institute for Clinical Systems Improvement, the National Guideline Clearinghouse database, and DynaMed. Search dates: August 12, 2020, and June 30, 2021.
The authors acknowledge Lauren Woldanski, MD, for her contributions to manuscript drafting and conceptual planning of this paper.