
The goal is not simply to avoid antibiotics but to use them in a judicious manner. Here are 10 steps family physicians, practices, and leaders can take.
Fam Pract Manag. 2021;28(6):15-20
Author disclosures: no relevant financial affiliations.

Writing an antibiotic prescription is a common task for family physicians. In ambulatory settings, approximately 20% of pediatric visits1 and 10% of adult visits2 result in an antibiotic prescription. While antibiotics can save lives, inappropriate or unnecessary use may exacerbate existing antibiotic resistance. According to the Centers for Disease Control and Prevention (CDC), nearly 3 million antibiotic-resistant infections and more than 35,000 consequent deaths happen each year in the United States.3
The CDC published its first Antibiotic Resistance Threats in the United States report in 2013, highlighting key bacterial and fungal threats and providing an action plan to combat their spread. Since then, awareness of antibiotic resistance has grown among health professionals, as well as the public. Antibiotic stewardship programs are now the norm in health care, though much of the focus remains on hospital-based interventions even though more than 60% of antibiotic expenses in the U.S. are incurred in outpatient settings.4
Family physicians are critical and well-positioned to lead the antibiotic stewardship movement for a few simple reasons. First, we write more antibiotic prescriptions than any other medical specialty — about one-fifth of all outpatient antibiotics prescribed each year.5 As the highest-volume prescribers of antibiotics, improving our prescribing habits would have significant health implications for our patients. Second, family physicians train and practice across multiple health care settings (e.g., ambulatory clinics, urgent care centers, nursing homes, and hospitals) in partnership with other specialty physicians. With such diverse roles and practice settings, family physicians are uniquely positioned to amplify the cultural change needed to curb antibiotic resistance. Finally, and perhaps most importantly, the relationship and trust between family physicians and their patients make the primary care visit an ideal venue to educate patients about antibiotic stewardship.
So how can we start improving antibiotic prescribing? This article offers a few suggestions for three key groups — individual family physicians, practices, and leaders.
KEY POINTS
While antibiotics can save lives, inappropriate or unnecessary use may exacerbate existing antibiotic resistance.
Family physicians are critical to antibiotic stewardship, in part because they write about one-fifth of all outpatient antibiotic prescriptions each year.
Family physicians can ensure appropriate use of antibiotics by leveraging clinical decision support applications, improving patient communication skills, offering delayed antibiotic prescriptions, and using standardized order sets, among other strategies.
STRATEGIES FOR INDIVIDUAL FAMILY PHYSICIANS
The goal of antibiotic stewardship is not simply to avoid antibiotics but to use them in a judicious manner. There are times when antibiotics are clearly needed without any diagnostic testing. Examples include a child with a high Centor score, a woman with low risk but a high pretest probability of a urinary tract infection, or a man with penile discharge and a history of high-risk sexual behavior. Additionally, some conditions such as acute rhinosinusitis or acute otitis media rely solely on clinical signs, symptoms, and history and have no currently available diagnostic testing. Unfortunately, there are a variety of circumstances when it is unclear to physicians and patients whether antibiotics are needed. In these instances, the following strategies can help.
1. Use point-of-care applications. When clinical uncertainty arises, family physicians can reach for internet-based and mobile app-based clinical decision support systems. (See “Point-of-care applications.”) Applications such as UpToDate and DynaMed boast intuitive search engines and hyperlinked topic outlines that make it easy to answer clinical questions during a brief patient encounter. For example, if a patient is diagnosed with acute bacterial rhinosinusitis, you might ask: What is the preferred management option? Per the CDC clinical decision support tool, “Watchful waiting is encouraged for uncomplicated cases for which reliable follow-up is available. Amoxicillin or amoxicillin/clavulanate is the recommended first-line therapy. Macrolides such as azithromycin are not recommended due to high levels of Streptococcus pneumoniae antibiotic resistance (~40%). For penicillin-allergic patients, doxycycline or a respiratory fluoroquinolone (levofloxacin or moxifloxacin) are recommended as alternative agents.”
2. Consult clinical guidelines. For busy physicians, difficulty keeping up with changing practice recommendations can be a barrier to appropriate antibiotic prescribing. To remain current with best practices, national clinical guidelines are by far the most exhaustive resource. A quality example is the skin and soft tissue infections guideline from the Infectious Diseases Society of America (IDSA). Additionally, the consensus guidelines on community-acquired pneumonia from the American College of Chest Physicians and IDSA are considered gold standards for this clinical topic. While incredibly comprehensive, these documents may be too cumbersome to navigate during a patient encounter. These guidelines may be far more useful during pre-visit planning for an unfamiliar case or post-visit research for a complex clinical picture when there is more time for reading before providing a recommendation.
3. Improve your communication. Patient expectations and patient satisfaction often compel physicians to inappropriately prescribe antibiotics. But patients are frequently not up-front about their expectations, and in trying to read between the lines physicians sometimes overestimate a patient's expectation for antibiotics. It is useful to draw out patients' views about antibiotics and address them directly.6,7 For patients who come to a visit expecting an antibiotic prescription, here are a few effective strategies that decrease antibiotic prescribing while maximizing patient satisfaction:
Use positive statements focused on specific guidance regarding symptom management (e.g., “A spoonful of honey can help the cough”), which are more effective than negative statements (e.g., “This is just a virus”), though a combination of both types of statements is most effective.8
Offer a contingency plan (e.g., close follow up and reassessment for possible antibiotics if the condition does not improve), which can help patients who expect antibiotics and do not receive them to feel significantly more satisfied.7
Choose your words carefully when describing the patient's clinical condition, as your choice of words may have significant implications on a patient's perception and satisfaction. For instance, deferring antibiotics for acute bronchitis may be far more palatable to patients when you refer to the condition as a “chest cold.”
Become more comfortable addressing patient expectations by practicing with difficult scenarios. A group of physicians in the Netherlands successfully prescribed fewer antibiotics for respiratory infections after undergoing communications skills training that included recorded encounters with standardized patients, peer feedback, and a shared decision-making seminar.9 While formal communication training may not be readily available, effective self-directed learning is possible. A good starting point is the following article: “How to Prescribe Fewer Unnecessary Antibiotics: Talking Points That Work with Patients and Their Families,” American Family Physician.
4. Use delayed antibiotic prescriptions. These prescriptions are given to patients with instructions to start later if symptoms persist (e.g., for mild acute otitis media or acute uncomplicated sinusitis). Interestingly, instructing patients to not fill until a later date vs. post-dating a prescription did not affect antibiotic fill rates in a study conducted in Canada.10 A 2017 Cochrane Review concluded that delayed prescriptions reduce the frequency of antibiotic use and preserve patient satisfaction without increasing harm.11 Critics argue that delayed prescribing does not improve patient outcomes.12 They also say it sends mixed messages (“Here's an antibiotic prescription even though your symptoms do not require it”). Yet in our current reality where many inappropriate prescriptions are written, delayed prescribing clearly reduces overall antibiotic use.
5. Adopt point-of-care testing (POCT). These tests are designed to provide rapid results that can help clinical decision making at the time of a patient's visit. Perhaps the most promising POCT for improving appropriate antibiotic use is the c-reactive protein (CRP) test. CRP is an inflammatory marker that increases to higher levels in bacterial infections compared with viral infections. Today, many European countries have included CRP POCT as an approved test in national guidelines when determining the need for antibiotics for management of respiratory tract infections. Unfortunately, CRP POCT is not yet approved for ambulatory use in the United States, though feasibility research is ongoing. Some evidence exists for procalcitonin as a tool to distinguish between bacterial and viral infections.13 It has seen increasing use in emergency departments and inpatient wards; however, feasibility and utility in primary care remain unclear due to the turnaround time for testing (20–30 minutes). A few procalcitonin algorithms to aid in decision making are available (see https://bmcmedicine.biomedcentral.com/articles/10.1186/s12916-017-0795-7), but it is unclear how beneficial they are in reducing antibiotic use and preventing unnecessary prescribing.
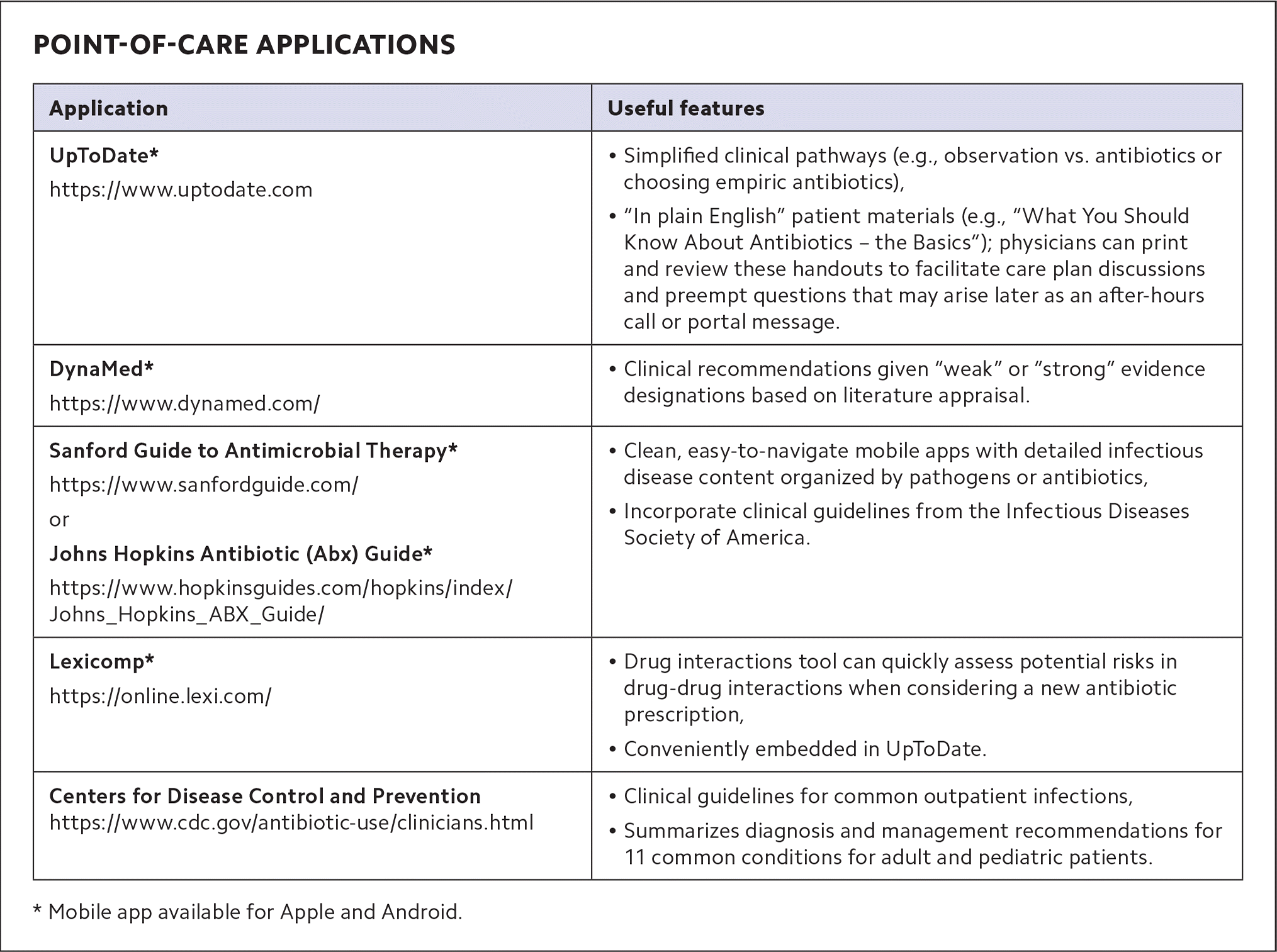
| Application | Useful features |
|---|---|
| UpToDate* |
|
| DynaMed* |
|
| Sanford Guide to Antimicrobial Therapy* or Johns Hopkins Antibiotic (Abx) Guide* |
|
| Lexicomp* |
|
| Centers for Disease Control and Prevention |
|
STRATEGIES FOR PRIMARY CARE PRACTICES
Antibiotic stewardship is not just the responsibility of physicians. To encourage the entire team to be engaged in the process, practices should consider the following.
1. Appoint a practice champion to lead the effort. Practices should identify a champion to direct antibiotic stewardship efforts, as similar roles have driven success in inpatient programs. Ideally, the champion should be clinically active, a person who understands the pressures of patient expectations and can credibly speakwith other clinicians who are improperly prescribing antibiotics. Champions should earn protected time and financial compensation for this work, a signal to team members just how much the practice values antibiotic stewardship.
Once a champion is appointed, the following framework may be a useful starting point to improve antibiotic stewardship in outpatient settings:
Set an annual stewardship goal. Practices may see the greatest benefit for their efforts by targeting prescriptions for acute bronchitis and viral upper respiratory infection, which are cited as the most frequent diagnoses associated with inappropriate antibiotic use.14
Implement evidence-based practice guidelines. Identify existing clinical guidelines that address the chosen goal and determine what specific protocols or training (e.g., communication skills training) may be helpful to prescribers and staff to achieve evidence-based clinical practice.
Educate all clinical staff about appropriate clinical practices. Make the goal clear to all staff, train all team members on any new clinical protocols, and publicize available resources (e.g., continuing medical education activities or access to experts) that support appropriate prescribing. (For additional staff-focused strategies, see the related article “Antibiotic Stewardship Throughout the Primary Care Visit: Opportunities for Office Staff.”)
Collect, analyze, and report data. This can be challenging to many practices, large or small. Those with limited resources may only be able to track and report a small subset of visits, while those in large health systems may need to overcome institutional inertia before getting access to any data. In either case, the ability to measure progress in real time may depend on having a champion who was an early EHR adopter at a small practice or one who can foster a relationship with medical informatics specialists at a health system.
2. Use consistent messaging. Setting patient expectations is critical in antibiotic stewardship. It relies heavily on clear and consistent messaging across all facets of a primary care practice that a patient interacts with. This requires establishing a standard, from the front-desk staff to all prescribing physicians, that visits involving viral infections will not result in antibiotic prescriptions. Practices should consider displaying posters or statements on a practice's website, waiting rooms, and exam rooms to reinforce the message about appropriate antibiotic use. Such displays have been shown to reduce antibiotic prescribing.15 To ease implementation, the CDC has created many handouts, posters, and infographics that are free for practices to use.
3. Provide individualized feedback. Personalized audits, peer comparison, and direct physician feedback are other proven strategies to help curb inappropriate prescribing.16,17 Ideally, physicians would receive periodic email reports of their antibiotic prescriptions, including comments about clinically inappropriate actions. These could be supplemented by peer comparisons, in which physicians would learn whether they are in the lowest decile for inappropriate prescribing rates or, if not, how their prescribing rates compare to the benchmarks set by their peers within the same region or health system. This strategy could also be paired with a clinician-led refresher course in evidence-based antibiotic prescribing and a review of available resources to aid clinical decision making.
STRATEGIES FOR PRIMARY CARE LEADERSHIP
Family physicians who are passionate about antibiotic stewardship should strongly consider leadership positions in health systems or departments of health. Traditionally, these roles have been occupied by inpatient physicians; however, a family medicine perspective in leadership is critical to developing agendas and policies that prioritize the needs of outpatient practices and facilities.
For family physicians in leadership roles, supporting front-line physicians should be their primary responsibility in antibiotic stewardship efforts. Here are a couple ways to do that.
1. Develop outpatient antibiograms. One example with high-impact potential is engaging local and regional laboratories to develop outpatient antibiograms. Antibiograms assess the local susceptibility and resistance of pathogens to a variety of antibiotics. Typically, antibiograms are tailored for emergency departments, inpatient wards, or intensive care units. Less commonly, they are made for outpatient settings. Access to outpatient antibiograms would enhance physicians' ability to prescribe appropriate and effective treatment for infections in their respective communities.
2. Leverage health IT. Leaders can also leverage widespread electronic health record (EHR) use to implement behavioral interventions that minimize inappropriate antibiotic prescribing. One approach is to create standardized order sets for common outpatient diagnoses that result in antibiotic prescriptions. Standardized order sets, crafted based on evidence-informed guidelines and local antibiograms, offer predetermined options for testing and medications based on the condition being treated. These order sets would help physicians stay current with best practices, reduce errors in antibiotic choice and dosing, and increase efficiency. To account for diversity in clinical risk factors and comorbidities among patients, these order sets would allow for alternative treatment regimens. In such cases, the EHR would prompt physicians to provide accountable clinical justification for bypassing the predefined choices.
With the rise of physician practice consolidation and widespread EHR adoption, efforts like those outlined above have the potential for significant uptake and benefit. Clearly, development of community-based antibiograms or electronic behavioral interventions require significant financial investment from departments of health or health systems. But such investment would undoubtedly affirm the leadership's commitment to antibiotic stewardship and the community's health.
ADDITIONAL RESOURCES
The Core Elements of Outpatient Antibiotic Stewardship: Facility Checklist. CDC.
A Field Guide to Antibiotic Stewardship in Outpatient Settings. CMS.
Nursing Home Antimicrobial Stewardship Guide. AHRQ.
Toolkit to Improve Antibiotic Use in Ambulatory Care (coming soon). AHRQ.
WHERE TO BEGIN
Success in antibiotic stewardship will require a multi-pronged strategy and commitment from all levels of the health care system. However, implementing many changes simultaneously may overwhelm physicians and staff and lead to burnout. A reasonable approach for cultural change is to assess readiness within a practice or organization and identify one or two interventions as the lowest-hanging fruits to implement. Changing the culture of antibiotic prescribing will take time, but small successes in the early stages will be key to building momentum.
