
Am Fam Physician. 1998;57(2):297-307
See related patient information handout on the implantable cardioverter-defibrillator, written by the authors of this article.
Implantable cardioverter-defibrillators are commonly used in patients who have life-threatening ventricular arrhythmias. With these implanted electronic devices, bradyarrhythmias and tachyarrhythmias can be recognized promptly and treated with electrical pacing, cardioversion or defibrillation. Implantable cardioverter-defibrillators have been shown to substantially reduce the incidence of sudden cardiac death in patients with known life-threatening ventricular arrhythmias. Their role in the primary prevention of sudden cardiac death in patients at high risk for ventricular arrhythmias is being evaluated. Technologic advances have allowed transvenous implantation of cardiac leads, obviating the need for open heart surgery and thereby lowering the risk of perioperative morbidity and mortality. Most electrical therapies are triggered appropriately to treat ventricular tachycardia/fibrillation. Inappropriate discharges may occur secondary to supraventricular causes of tachycardia, environmental interference from electromagnetic devices or malfunction of the cardioverter-defibrillator. All episodes of discharge merit investigation. With recurrent or frequent discharges, prompt evaluation and hospitalization are often necessary.
Implantable cardioverter-defibrillators have been used to treat patients with life-threatening ventricular arrhythmias since 1980.1 Improvements in technology have simplified implantation and increased the electrical therapies that can be delivered with these devices.2 Consequently, cardioverter-defibrillators are being used frequently in patients with serious ventricular arrhythmias.
Because of the increased prevalence of implantable cardioverter-defibrillators, many family physicians are, or will be, providing primary care to patients with these implants. Thus, family physicians need to know how the devices work so that they can answer patients' questions and make appropriate referrals if problems occur.
Components and Implantation
The implantable cardioverter-defibrillator is an electronic device consisting of a generator and a lead system. The purposes of the device are to monitor heart rhythm and treat detected abnormal heart rhythms using variable modalities.
Most cardioverter-defibrillators currently in use are implanted using transvenous technology. Implantation no longer requires open heart surgery and placement of electrical patches on the ventricle. However, devices that employ defibrillation patches on the ventricle are still in use, and these leads are usually retained when a generator is upgraded. Improvements in generator technology have increased the options for treating tachyarrhythmias. These options now include electrical therapy (pacing), which is used to treat bradyarrhythmias. Thus, sustained ventricular tachycardia can be treated with competitive (overdrive) pacing or synchronized cardioversion, ventricular fibrillation can be treated with defibrillation, and bradycardia can be treated with pacing.
Current implantable cardioverter-defibrillators store information about the arrhythmias. When retrieved, this information can assist the physician in determining if cardioverter-defibrillator use was appropriate in treating a rhythm disturbance.
Detection of Arrhythmias
All implantable cardioverter-defibrillators use heart rate as the main criterion for detecting abnormal heart rhythms and for employing decision algorithms to determine the therapeutic response once an arrhythmia is detected. The devices can be programmed for the heart rate cutoff at which the implantable cardioverter-defibrillator considers a heart rhythm abnormal, as well as the number of consecutive beats of tachycardia before therapy is initiated. Most implantable cardioverter-defibrillators incorporate multiple tiers of heart rate thresholds that can be set up to treat bradycardia, slow ventricular tachycardia, fast ventricular tachycardia and ventricular fibrillation.
Since the devices may have difficulty in differentiating one type of fast heart rhythm from another, inappropriate electrical therapy may be given for atrial fibrillation with a rapid ventricular response or sinus tachycardia associated with exercise. Programming a device for individual patient characteristics can help to decrease the occurrence of inappropriate shocks or pacing. Most devices also have the ability to analyze the cardiac electrical waveform and use this analysis in combination with rate detection to avoid the administration of inappropriate electrical therapies. The addition of a dual-chamber cardioverter-defibrillator has made the detection of, and the avoidance of inappropriate therapies for, supraventricular arrhythmia more precise.
Treatment of Arrhythmias
Implantable cardioverter-defibrillators can treat abnormal heart rhythms using pacing therapy, synchronized cardioversion or defibrillation. If the heart rate falls below a programmed rate, the devices provide ventricular demand pacing (Figure 1). Pacemaker-dependent patients or patients with frequent episodes of bradycardia commonly have a bradycardia pacemaker implanted in addition to the cardioverter-defibrillator. This dual implantation diminishes battery drain on the cardioverter-defibrillator and also provides dual-chamber (atrium and ventricle) pacing capability. However, implantable cardioverter-defibrillator technology that allows dual-chamber pacing is now available and should obviate the need for separate pacemakers and defibrillators.3
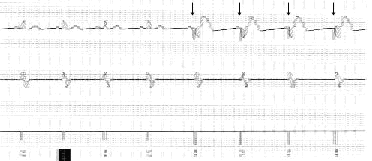
Ventricular tachyarrhythmias are commonly treated with a step-wise approach that may include competitive pacing and low- to high-energy synchronized cardioversion. Competitive pacing uses electrical pacing of the ventricle at a faster rate than the ventricular tachycardia to interrupt the reentry circuit, thus terminating the abnormal rhythm (Figure 2). This therapy makes it less necessary to treat ventricular tachycardia with potentially painful discharges of the implantable cardioverter-defibrillator in the conscious patient.2 It also minimizes battery depletion in the device. Frequently, the patient is unaware that an abnormal rhythm occurred and was treated with pacing. Since ventricular tachycardia may accelerate with attempts at competitive pacing, the ability to cardiovert or defibrillate is necessary (Figure 3).
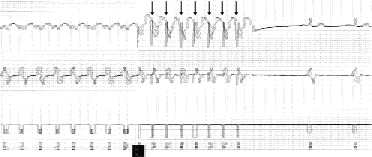

If competitive pacing fails or has not been programmed into the device because it is known to be ineffective in the particular patient, cardioversion is often the next programmed therapy. Cardioversion delivers a synchronized high-voltage discharge through the lead system for the purpose of depolarizing a sufficient mass of the myocardium to stop the ventricular tachycardia. In general, cardioversion exceeding 1 to 2 joules (J) results in associated skeletal and diaphragmatic muscle depolarization and thus is painful to the conscious patient. High-energy discharge (commonly 10 to 40 J) delivered asynchronously (defibrillation) is used to treat rapid ventricular rates consistent with ventricular fibrillation.
If a delivered electrical therapy fails to terminate an abnormal rhythm, successive therapies are delivered. If the abnormal rhythm is not terminated after four to six therapies, no further treatment is given.
Technology of the Lead System
The lead system connects the generator of the implantable cardioverter-defibrillator to the heart. This system allows heart rate to be detected and electrical therapies to be delivered. Lead technology has progressed rapidly in the past 10 years (Figures 4 and 5), so that most cardioverter-defibrillators now require only a single lead that can be placed transvenously.4 Since the generator forms one electrical pole of the cardioversion-defibrillation circuit, a second lead is not needed.


With older systems, the generator box was usually implanted in an abdominal wall pocket. However, current systems are most often implanted in the left pectoral area, with venous access to the heart obtained through the left subclavian or cephalic vein, similar to the access used for a pacemaker (Figures 6 and 7).
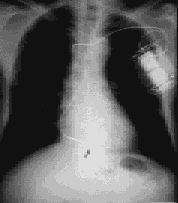
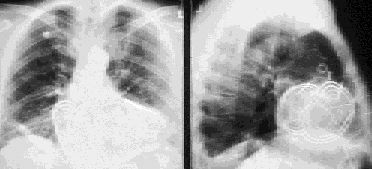
Implantation Approach and Complications
Perioperative mortality for implantation of current cardioverter-defibrillator systems is less than 1 percent.5 Perioperative morbidity is low but may include exacerbation of ventricular tachycardia or fibrillation, postoperative atrial fibrillation and complications related to lead placement, including pneumothorax, cardiac perforation and venous thrombosis. Early lead dislodgment can occur and requires repositioning of the lead system. Local hematoma or infection at the implantation site may also occur. Patients receiving a pectorally implanted converter-defibrillator are commonly sent home the day after implantation if no complications have occurred and a chest radiograph shows that the lead is in the correct position.
Efficacy of Implantable Cardioverter-Defibrillators
Therapy using implantable cardioverter-defibrillators has significantly diminished the risk of sudden cardiac death in patients with known life-threatening ventricular arrhythmias at high risk for recurrence. In several trials,5–8 the mortality rate from sudden cardiac death was less than 2 percent after one year and 10 percent after five years. While comparison with historical data is difficult, recurrent sudden cardiac death rates as high as 20 percent at one year and 35 percent at three years were reported in the mid-1970s9 to early 1980s.10 While more recent antiarrhythmic drug trials have reported reduced rates of sudden cardiac death,11,12 these rates remain higher than those reported for implantable converter-defibrillators.5–8
Many cardiac specialists believe that implantable cardioverter-defibrillators are superior to antiarrhythmic drugs in decreasing overall cardiac and total mortality rates in patients with known life-threatening ventricular arrhythmias. This belief was confirmed with the recent publication of the Antiarrhythmics versus Implantable Defibrillator (AVID) trial.13 In this trial, 1,016 patients with symptomatic ventricular tachycardia or ventricular fibrillation were randomized to receive either an implantable cardioverter-defibrillator (507 patients) or antiarrhythmic drug therapy (509 patients), primarily empiric amiodarone therapy.14 Follow-up was complete in 1,013 patients, with the cardioverter-defibrillator group showing improved unadjusted survival at one year (89.3 percent versus 82.3 percent), two years (81.6 percent versus 74.7 percent) and three years (75.4 percent versus 64.1 percent). The statistical significance was P < 0.02. Based on the results of the AVID trial, the implantable cardioverter-defibrillator is considered the preferred treatment in patients with documented symptomatic ventricular tachycardia or ventricular fibrillation.
A trial similar to AVID is under way in Canada.15 Although patient recruitment has ended, no results have been reported.
Several clinical trials are exploring the use of implantable cardioverter-defibrillators for the primary prevention of sudden cardiac death in high-risk patients who have cardiac dysfunction associated with ischemic or nonischemic heart disease. The Multicenter Automatic Defibrillator Implantation Trial (MADIT)16 evaluated patients who had experienced a myocardial infarction and had nonsustained ventricular tachycardia and cardiac dysfunction (a left ventricular ejection fraction of less than 35 percent). In these patients, electrophysiologic studies revealed inducible sustained ventricular tachycardia that was unresponsive to intravenously administered procainamide. The patients were randomized to receive either an implantable cardioverter-defibrillator or conventional antiarrhythmic therapy, primarily amiodarone. The total mortality rate decreased in the patients who received the implantable cardioverter-defibrillator.
Based on the MADIT results, the U.S. Food and Drug Administration has approved the use of implantable cardioverter-defibrillators in this high-risk population. Several ongoing trials are addressing the potential benefits of the prophylactic implantation of these devices in other high-risk patient populations.
Indications
Indications for the use of implantable cardioverter-defibrillators have been specified by a joint task force of the American College of Cardiology and the American Heart Association (Table 1).17 Since the task force's report was published in 1991, electrophysiologic studies are being used less frequently to test antiarrhythmic drug efficacy. Studies10,11 have shown that empiric treatment with amiodarone or Holter-guided antiarrhythmic drug efficacy may be equal to or better than prediction of antiarrhythmic drug efficacy guided by electrophysiologic study. Based on the results of the AVID trial, early treatment with an implantable cardioverter-defibrillator will become a mainstay of treatment in patients with serious ventricular arrhythmias.
Treatment using an implantable cardioverter-defibrillator is expensive; the device, implantation procedure and hospital stay cost $50,000 to $60,000. However, advances in technology and implantation techniques appear to be reducing the cost of treatment.18
Estimates of the cost of implantable cardioverter-defibrillators relative to years of life saved in the secondary prevention of sudden cardiac death approach $50,000, which is similar to the cost of other life-saving procedures, such as hemodialysis in renal failure.19 Cost analysis data from the AVID trial are not yet available.
General Management of Patients
Implantation of a cardioverter-defibrillator is becoming an increasingly straightforward procedure, but careful follow-up remains important to ensure optimal function. In general, the patient with a newly implanted cardioverter-defibrillator is seen one week after implantation for a wound check and “interrogation” of the device. This visit is usually with the physician who placed the device.
Thereafter, the patient is seen every three to four months by a cardiac electrophysiologist. During each check-up, the device is interrogated. This interrogation is typically done in a specialized pacemaker-defibrillation follow-up clinic run by the cardiac electrophysiologist and a trained technician or nurse.
Interrogation of the cardioverter-defibrillator is done by communicating via electromagnetic coupling with a “wand” placed over the device and attached to a programmer. The programmer is specific to the device of each manufacturer. Interrogation allows the physician to determine which electrical therapies have been given. Lead integrity and battery status are also checked. The device can then be adjusted to optimize detection and therapy parameters. Most cardioverter-defibrillators record the patient's electrocardiographic tracing at the time of arrhythmia detection. This information can be analyzed at follow-up visits to determine the nature of the arrhythmia and the efficacy of the electrical therapy that was given.
Patients who have a cardioverter-defibrillator may present to their family physician with questions about the devices, particularly following a discharge. Thus, family physicians need to be sufficiently familiar with the basic aspects of cardioverter-defibrillator function to answer these questions appropriately and consult with a cardiac electrophysiologist when necessary.
Environmental Interference
Communication with an implantable cardioverter-defibrillator is accomplished via electromagnetic coupling. Inadvertent exposure to magnetic fields may interfere with the appropriate functioning of the device, lead to inappropriate discharges or cause the device to reprogram. In general, environmental interference that affects pacemakers may have similar effects on implantable cardioverter-defibrillators.
In the Home. In the average home, it would be uncommon, but not impossible, to encounter appliances that have a magnetic field strong enough to interact with an implantable cardioverter-defibrillator. Small kitchen appliances (including microwave ovens) and small power tools and motors do not interfere with cardioverter-defibrillators if they are properly grounded and factory-installed shielding has not been modified. Rarely, inappropriate discharges of implantable cardioverter-defibrillators have been caused by such items as an electrical razor20 and a toy car with a hand-held radiofrequency remote control.21 In at least some situations, abnormalities have been detected in the lead system of the implantable cardioverter-defibrillator.
Electrical signals from digital cellular telephones may be sensed as an abnormal heart rhythm by implantable cardioverter-defibrillators, and inappropriate therapies may be applied.22 The more common analog cellular telephones do not appear to cause such a problem.
In general, interaction of any small electromagnetic device with an implantable cardioverter-defibrillator requires that the two devices be in close proximity, usually within 10 cm of each other. The greater the output power of a given device, the greater the possibility of an interaction. If an implantable cardioverter-defibrillator discharge occurs when an electromagnetic device is being used, cessation of use and investigation of potential interaction are warranted.
In the Workplace. Large electrical motors and industrial arc welders can interfere with the appropriate functioning of implantable cardioverter-defibrillators or lead to inappropriate discharges of the devices.23 In general, questions concerning a work environment that has industrial motors or arc welding equipment should be referred to a cardiac electrophysiologist. After testing of the potential interactions between the cardioverter-defibrillator and the workplace machinery, it may be possible for the patient to return to work.
In the Hospital. The hospital environment may well have the greatest potential for exposure to devices that can interfere with the appropriate functioning of implantable cardioverter-defibrillators. Magnetic resonance imaging, extracorporeal shock wave lithotripsy, surgical or endoscopic electrocautery and peripheral nerve stimulators have all been implicated as causes of abnormal functioning in implantable cardioverter-defibrillators or pacemakers.24–27
Patients with implantable cardioverter-defibrillators who require these diagnostic or therapeutic modalities should be referred to a cardiac electrophysiologist for evaluation of the feasibility of the particular test or procedure. These modalities may be used in selected patients, but the cardioverter-defibrillator should be evaluated both before and after the test or procedure.
Evaluation of Discharges
At least one spontaneous discharge occurs in about one half of patients during the first year after implantation of a cardioverter-defibrillator. Thereafter, the incidence of spontaneous discharge declines.28 In most instances, spontaneous cardioverter-defibrillator discharges are appropriate and treat the abnormal rhythm for which the device was implanted. However, inappropriate discharges occur at some time in 10 to 30 percent of patients with implantable cardioverter-defibrillators.29 The causes of these inappropriate discharges vary (Table 2).
| Etiology of inappropriate therapy | Clues typical for the etiology | Possible solutions by the electrophysiologist | |
|---|---|---|---|
| Supraventricular source of tachycardia meeting the implantable cardioverter-defibrillator rate cutoff: | |||
| Sinus tachycardia | Arrhythmia occurs with physical exertion | Reprogram the implantable cardioverter-defibrillator | |
| Start beta-blocker therapy | |||
| Paroxysmal supraventricular tachycardia | History of paroxysmal supraventricular tachycardia | Start antiarrhythmic drug therapy or radiofrequency ablation therapy | |
| Atrial fibrillation/flutter with rapid ventricular response | History of atrial rhythm disturbance | Reprogram the implantable cardioverter-defibrillator | |
| Start antiarrhythmic drug therapy or radiofrequency ablation therapy | |||
| Environmental interference from equipment in the home, workplace or hospital | Patient is near the suspected electromagnetic field source at the time of implantable cardioverter-defibrillator discharge | Avoid exposure to electromagnetic source or provide improved shielding | |
| Interaction of pacemaker and implantable cardioverter-defibrillator | Patient has both implanted devices | Evaluate interaction | |
| Consider reprogramming either the implantable cardioverter-defibrillator or the pacemaker | |||
| Malfunction of the implantable cardioverter-defibrillator | No symptoms before discharge | Evaluate the lead and generator with a chest radiograph | |
| Discharge reproduced with arm movement | Test the device with movement | ||
| Commonly must replace the sensing lead of the device | |||
| “Phantom shock” (hypnagogic muscle contraction interpreted by the patient as implantable cardioverter-defibrillator discharge) | Occurs at night, usually while drifting off to sleep | No event recorded by the implantable cardioverter-defibrillator | |
| Reassure patient | |||
Most of the current implantable cardioverter-defibrillators cannot completely differentiate supraventricular causes of tachycardia (e.g., sinus tachycardia or atrial fibrillation) from ventricular tachycardia. For this reason, inappropriate discharges can occur. Lead failure, the most common device-related malfunction, can also result in inappropriate discharge.
In the so-called “phantom shock,” the patient feels the sensation of a discharge when one has not occurred. Phantom shocks are relatively common. They frequently occur when a patient is drifting off to sleep, and they may be associated with hypnagogic muscle contractions occurring in conjunction with sleep onset or myoclonus.
Interrogation of an implantable cardioverter-defibrillator after any discharge allows evaluation of the stored arrhythmia event. This can be helpful in determining whether and why a discharge occurred. Through an evaluation of the heart rate just before the onset of arrhythmia detection and again after electrical therapy was delivered, it is often possible to determine whether the therapy was appropriate or inappropriate. External electrical noise that the implantable cardioverter-defibrillator inappropriately senses as an arrhythmia can be discovered by its typical appearance on the electrocardiographic tracing.
Patients who experience a discharge of their implantable cardioverter-defibrillator may first contact their family physicians. The patient who has experienced a single discharge usually does not need to be evaluated as an emergency, even if the episode was associated with symptoms. Often, information about the discharge can be relayed to the cardiac electrophysiologist, and an appointment can be set up to interrogate the implantable cardioverter-defibrillator to determine the appropriateness of the discharge and whether the device requires programming changes or drug therapy is needed.
In contrast, repeated discharges or symptoms suggestive of an ongoing arrhythmia (i.e., palpitations, syncope, presyncope) should prompt a rapid evaluation and, often, hospitalization of the patient. Most patients who have an implanted cardioverter-defibrillator always carry with them information about their implant model, as well as the name of their cardiac specialist.
Prompt electrocardiographic monitoring is required in a patient who is having repeated discharges of the implantable cardioverter-defibrillator. If it can be determined that the discharges are occurring for an inappropriate reason, deactivation of the device may be warranted.
Deactivation of all current implantable cardioverter-defibrillators can be accomplished by placing a donut magnet over the generator and taping it in place. Donut magnets are commonly available in emergency departments or coronary care units. Deactivation of the device by the donut magnet suspends tachyarrhythmia therapy but does not affect bradycardia pacing. Magnet placement in some implantable cardioverter-defibrillators can disable tachyarrhythmia therapy permanently, despite removal of the magnet. Therefore, deactivation of an implanted cardioverter-defibrillator is most safely accomplished in a setting where it is possible to treat new tachyarrhythmias with external cardioversion-defibrillation.
Additional therapy, commonly intravenously administered antiarrhythmic drugs, is indicated in patients who are having ongoing discharges when electrocardiographic monitoring is consistent with appropriate therapy for repetitive ventricular tachycardia or fibrillation. Temporary suspension of tachyarrhythmia therapy may be appropriate in some patients if the arrhythmia is tolerated hemodynamically. Patients with recurrent discharges for either appropriate or inappropriate reasons should be evaluated by a cardiac electrophysiologist.