
Am Fam Physician. 1998;58(2):351-352
to the editor: Varicella affects several million U.S. residents annually and is usually a self-limiting illness. Adults have higher morbidities and mortalities than children. Immigrants from tropical and subtropical climates are less likely to be infected as children, thereby resulting in greater susceptibility as adults. Increased use of varicella vaccine in children and susceptible adults, particularly in the immigrant population, will decrease the number of cases and should also decrease the associated morbidity and mortality.
We present the case of a Guaynese man who had resided in the United States for 14 years and who was the fifth person in his household to contract varicella. The index case was his four-year-old granddaughter who had been exposed to a preschool friend. Subsequently, his 37-year-old son, his two-year-old grandson and his 28-year-old daughter contracted the illness before he did. All of the cases were limited to cutaneous involvement, with the exception of the son, who died as a result of pneumonitis. None of the patients received antiviral therapy before hospitalization.
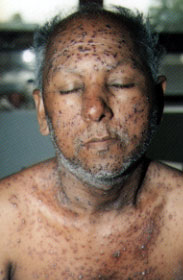
Our patient was a 61-year-old man with a history of diabetes mellitus who had pain on swallowing and a vesicular rash on the abdomen, extremities and face. On day 3 of his illness, he was diagnosed as having uncomplicated varicella. He was afebrile, had an extensive vesicular rash (see figure) and had a normal chest radiograph. He was admitted two days later for intravenous administration of acyclovir, 10 mg per kg every eight hours. On day 3 of hospitalization, he had multiple episodes of diarrhea. Stool evaluation was negative and findings on sigmoidoscopy were unremarkable. The diarrhea was thought to be secondary to acyclovir therapy and responded to treatment with Imodium. The patient did well with treatment and was discharged on day 12 of his illness.
Before the introduction of varicella vaccine in 1995, about 4 million cases of varicella occurred yearly in the United States, with 4,000 to 9,000 hospitalizations and 100 deaths per year in nonimmunocompromised persons. Although less than 5 percent of varicella cases occur in adults, patients over 20 years of age account for more than one half of the fatalities related to varicella.1 The Centers for Disease Control and Prevention (CDC) reported three deaths in 1997 in young women exposed to varicella by their unvaccinated preschool-aged children. The CDC reemphasized the use of varicella vaccine, varicella immune globulin when indicated and antiviral therapy.
Varicella vaccine was approved by the U.S. Food and Drug Administration in March of 1995 for use in healthy persons one year of age and older. The American Academy of Pediatrics, the American Academy of Family Physicians and the Advisory Committee on Immunization Practices of the U.S. Public Health Service have recommended a strategy of universal immunization of children 12 to 18 months of age. Older susceptible persons should also be vaccinated, especially those who are in close contact with persons at risk of developing serious varicella-related complications (i.e., health care personnel and contacts of immunocompromised persons). The vaccine is not approved for use in pregnant women or in immunocompromised patients.
The best defense for persons who cannot be vaccinated is to immunize those contacts likely to expose them to varicella. Varicella zoster-immune globulin can reduce the severity of symptoms when administered to individuals within 72 hours of exposure. It is indicated for those at high risk for complications: newborns whose mothers develop varicella within four days before and up to two days after delivery; healthy susceptible adults; and children and adults who are immunosuppressed due to their primary disease (malignancies, infection with human immunodeficiency virus) or secondary to therapy (steroids, chemotherapy, etc.).
Oral acyclovir has been shown to decrease the severity of varicella in children if given within 24 to 48 hours after the onset of rash.1 Adverse effects of acyclovir include neurologic abnormalities (encephalopathy, tremors) and renal abnormalities.2 Gastrointestinal disturbances, such as nausea, vomiting and bloating, have been reported.3 Diarrhea has been described in one series with diarrhea of one to four days' duration developing in 52 (35 percent) of 148 patients receiving acyclovir.3
This case highlights the need to initiate varicella immunizations in immigrant populations, as well as the need to administer antiviral therapy as soon as possible in the adult population. Vaccination might have prevented varicella infection in the children and subsequent exposure in the adults. Physicians should be aware of the higher risk of varicella susceptibility in patients from warmer climates and the need to initiate antiviral therapy as soon as possible in the adult population.