
Am Fam Physician. 1999;59(10):2828-2834
This is Part II of a two-part article on stroke. Part I, “A Clinical Update on Prevention,” appeared in the last issue (Am Fam Physician 1999;59:2475–82.
Optimal treatment of the patient who has sustained an acute ischemic stroke requires rapid assessment and early intervention. The leisurely approach to acute stroke management sometimes taken in the past should be replaced by an approach that treats stroke as a true medical emergency. Thrombolysis with tissue plasminogen activator has been labeled for the treatment of acute ischemic stroke, but it must be given within three hours of stroke onset. However, fibrinolytic therapy can be given safely to only a fraction of patients with acute stroke, and more broadly applicable therapies are needed. Recent evidence does not support the routine use of heparin in patients with acute stroke, and early use of aspirin offers only modest benefit. Neuroprotective therapies designed to interfere with cytotoxic events initiated by ischemia are undergoing clinical trials that should be completed within the next year. At present, only tissue plasminogen activator has been labeled for acute stroke treatment; however, other agents are on the horizon, and much can be done supportively to improve neurologic outcome. Because of the unique susceptibility of neurons to ischemia, minutes count. Thus, hospitals providing care for patients with acute stroke should organize clinical protocols and pathways for effective implementation of therapies.
Stroke continues to have a devastating impact on public health, and it remains a leading cause of death and disability in the United States. At least 700,000 new stroke cases occur every year.1 Approximately 85 percent of strokes are ischemic in nature.2 Although the incidence of ischemic stroke has declined over the past 20 years, the mean age of the population has risen, resulting in a continual increase in the absolute number of strokes. Recent projections indicate that by the year 2050, more than 1 million strokes will occur each year in the United States. In this second part of our two-part article, we review recent advances in the management of acute ischemic stroke (Table 1). Advances in stroke prevention were considered in the first part of our article.3
| Systematic approach to stroke management (i.e., stroke units, clinical pathways) improves outcome. |
| Early initiation of aspirin following acute ischemic stroke offers modest long-term benefits. |
| Tissue plasminogen activator, if given within three hours of onset of ischemic stroke, improves neurologic outcome.* |
Neuronal Death in Stroke
Neurons die within a few minutes of oxygen deprivation. Thus, some neuronal death occurs in areas of no blood flow within minutes of stroke onset. Around such areas of necrosis exist regions of hypoperfused, electrically silent tissue that barely receives enough blood flow to keep neurons alive. This tissue area is called the “ischemic penumbra.” A major goal of acute stroke management is resuscitation of the ischemic penumbra. If reperfusion of the penumbra occurs expeditiously, neurons recover and the patient improves; with no reperfusion, a time-related attrition converts ailing neurons to frank infarction.
Because of the time-dependent death of neurons in the ischemic penumbra, emphasis should be placed on the earliest possible intervention. With the availability of new interventions, minutes count, and the leisurely approach to acute stroke often taken in the past should be replaced by an approach that treats stroke as a true emergency. To facilitate the earliest possible treatment, the public (especially persons at high risk for stroke) must be educated to call “911” when symptoms of stroke first appear.4
Ancillary Treatment
Elevated Blood Pressure. Unless systolic blood pressure exceeds 220 smm Hg or diastolic pressure exceeds 120 mm Hg (sustained on repeated measurement), elevated blood pressure should not be treated within the first days after ischemic stroke. The ischemic penumbra loses autoregulation, and perfusion is directly linked to mean arterial pressure. Acute elevations in blood pressure are often transient, and spontaneous declines are common. Overzealous treatment of hypertension following acute ischemic stroke can convert the ischemic penumbra into an infarct. The two exceptions to this general recommendation are as follows: (1) after use of tissue plasminogen activator (t-PA), blood pressure should be maintained below 185/110 mm Hg, and (2) in the presence of myocardial infarction, heart failure or aortic dissection, elevated blood pressure should be treated aggressively. If antihypertensive therapy is necessary, agents that have a rapid onset and predictable response should be used (Table 2).5
Fever. In patients with acute stroke, fever is not uncommon. Whatever the cause, fever should be suppressed in these patients. In experimental models of brain ischemia as well as in clinical studies,6 even mild elevations in body temperature consistently worsen the neurologic outcome from ischemic insults.
Hyperglycemia. In the setting of acute stroke, hyperglycemia may be deleterious to the ischemic penumbra by permitting anaerobic metabolism with the creation of local lactic acidosis. It has not been shown that control of glucose improves stroke outcome in humans; however, based on observational and experimental studies, the general consensus is that glucose levels should be kept below 150 mg per dL (8.3 mmol per L).
Acute Stroke Units. Based on a meta-analysis of randomized trials, acute stroke units appear to be associated with better long-term outcome and lower mortality than regular hospital care.7 Admitting 1,000 stroke patients to a dedicated stroke unit would prevent about 20 deaths over the course of one year.7 However, many of the studies on this subject were conducted in Europe some years ago, with mean hospital stays of several weeks, and the applicability of the findings to current practice in the United States is undetermined. Whether or not a designated stroke unit is available, an organized, systematic approach to stroke management (using clinical pathways and algorithms, and monitoring indices of quality) will likely improve outcome. Hospitals providing care to patients with acute stroke should consider organizing stroke teams to facilitate a more systematic approach.
Pharmacologic Interventions
More than two dozen agents and interventions are currently undergoing clinical testing for use in the treatment of acute ischemic stroke. The only specific intervention validated by adequate clinical trials and labeled for this use by the U.S. Food and Drug Administration (FDA) is t-PA (given within three hours of stroke onset). However, the results of ongoing clinical trials may soon expand available therapeutic options.
Tissue Plasminogen Activator. The results of the National Institute of Neurologic Disorders and Stroke (NINDS, a section of the National Institutes of Health [NIH]) t-PA trial were published in December 1995.8 The FDA granted labeling approval to t-PA in June 1996. The NINDS study showed that the use of t-PA within three hours of ischemic stroke onset substantially improved long-term functional outcome compared with placebo, even when the approximately 6 percent incidence of intracerebral hemorrhage in t-PA recipients was considered. Based on this trial, for every 100 patients given t-PA, 12 more experience complete neurologic recovery than with placebo (Table 3).8 The risk of intracerebral hemorrhage increased significantly in patients treated with t-PA when early infarction changes were present in the computed tomographic (CT) scan and in patients with a high NIH Stroke Scale at baseline.9 The European Cooperative Acute Stroke Study (ECASS)10 randomized trial of t-PA using a six-hour time limit to treatment and a slightly higher dose of t-PA did not demonstrate an overall benefit, primarily because of a high rate of brain hemorrhage. Thus, at present, the three-hour maximum time from stroke onset to treatment must be strictly observed. A follow-up trial from the European investigators (ECASS II) tested a lower dose of t-PA (0.9 mg per kg) in the three- to six-hour window and excluded patients with early CT changes of infarction involving one third or more of the cerebral hemisphere. The results showed no significant benefit for patients treated with t-PA, although the incidence of t-PA–related intracerebral bleeding was similar to that found in the NINDS trial.11
The following points are worth emphasis:
Therapy with t-PA for acute ischemic stroke is effective and of overall benefit, but it carries risk. About one in 15 recipients suffers serious brain hemorrhage, even if t-PA is used according to strict guidelines.
The t-PA dose is given intravenously over one hour; no arteriography is required.
The t-PA dose must be given within three hours of stroke onset.
When a patient awakens from sleep with a neurologic deficit, onset of stroke must be assumed to be the time that sleep commenced.
It is sometimes difficult to be certain of the exact time of stroke onset at initial evaluation; if time of onset is uncertain, t-PA should not be given.
The CT scan must document the absence of intracranial bleeding before treatment. (Some authorities also recommend that t-PA not be given if the CT scan shows any evidence of early infarction.)
Patients with severe ischemic strokes have a higher risk of t-PA–associated brain hemorrhage, but they also have the most to gain.9
| Outcome | t-PA (%) | Placebo (%) | Difference (%) | P value |
|---|---|---|---|---|
| Favorable outcome at three months | 41 | 29 | +12 | 0.008 |
| Mortality at three months | 17 | 21 | –4 | NS |
| Symptomatic intracerebral hemorrhage at 36 hours | 6 | 1 | +5 |
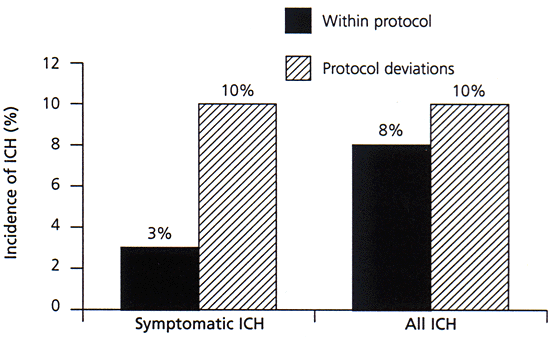
Substantial controversy has surrounded recommendations for widespread clinical use of t-PA, despite FDA labeling.12,13 Although many American stroke experts advocate t-PA use according to strict guidelines, most European authorities believe that general clinical use is premature.14 Those opposing the general use of t-PA note that only a single clinical trial, carried out in specialized stroke research centers, has shown overall benefit, whereas other trials of thrombolytic agents have consistently shown no benefit or even harm.14 Advocates of t-PA counter that the specific agent (i.e., t-PA instead of streptokinase), the dose and the three-hour maximum time-to-treatment explain the difference from negative trials. A recent study showed that the use of t-PA in community hospitals is feasible and safe as long as the American Heart Association (AHA) guidelines and NINDS protocol are followed (Figure 1).15 Use of t-PA for acute ischemic stroke according to strict guidelines has been endorsed by the AHA Stroke Council and the American Academy of Neurology.
Intravenous t-PA is not difficult to administer, but it requires strict adherence to eligibility criteria (Table 4),16 emergency CT scanning capabilities with experienced interpretation, and an initial 24 hours of patient monitoring in an intensive care unit. Although t-PA is expensive (approximately $2,000 per treatment or dose), cost-effectiveness analysis of the results of the NINDS t-PA trial indicate substantial long-term savings because fewer patients receiving t-PA require chronic care. Given the three-hour time window (which eliminates almost all patients who awaken with stroke), only a fraction of patients with acute ischemic stroke are eligible for t-PA treatment. Education of the public (“dial 911 for stroke”) and streamlining of the emergency evaluation of stroke patients can increase the fraction of patients with acute stroke who are eligible to receive t-PA. Physicians considering the use of t-PA in patients with acute ischemic stroke are urged to review the AHA guidelines and to adhere strictly to the NINDS t-PA protocol guidelines.16
| Inclusion criteria | |
| Age greater than 18 years | |
| Clinical diagnosis of ischemic stroke, with onset of symptoms within three hours of initiation of treatment | |
| Noncontrast CT scan with no evidence of hemorrhage | |
| Exclusion criteria | |
| History | |
| Stroke or head trauma in previous three months | |
| History of intracranial hemorrhage that may increase risk of recurrent hemorrhage | |
| Major surgery or other serious trauma in previous 14 days | |
| Gastrointestinal or genitourinary bleeding in previous 21 days | |
| Arterial puncture in previous seven days | |
| Pregnant or lactating patient | |
| Clinical findings | |
| Rapidly improving stroke symptoms | |
| Seizure at onset of stroke | |
| Symptoms suggestive of subarachnoid hemorrhage, even if CT scan is normal | |
| Persistent systolic pressure greater than 185 mm Hg or diastolic pressure greater than 110 mm Hg, or patient is requiring aggressive therapy to control blood pressure | |
| Clinical presentation consistent with acute myocardial infarction or postmyocardial infarction pericarditis requires cardiologic evaluation before treatment | |
| Imaging results | |
| CT scan with evidence of hemorrhage | |
| CT scan with evidence of hypodensity and/or effacement of cerebral sulci in more than one third of middle cerebral artery territory | |
| Laboratory findings | |
| Glucose level less than 50 mg per dL (2.8 mmol per L) or greater than 400 mg per dL (22.2 mmol per L) | |
| Platelet count less than 100,000 per mm3 (100 × 109 per L) | |
| Patient is taking warfarin and has abnormal International Normalized Ratio | |
| Patient has received heparin within 48 hours, and partial thromboplastin time is elevated | |
Streptokinase. Based on clinical trials to date, intravenous streptokinase has not been of overall benefit in patients with acute stroke, but it is uncertain whether the difference from the t-PA results reflects the longer time window in these trials (i.e., up to six hours after stroke onset), the doses tested or the specific agent.14 Intra-arterial streptokinase, urokinase and prourokinase are being tested in ongoing clinical trials. Pending further data, these agents should probably not be used outside of research protocols. Ancrod is a fibrinogenolytic agent that has been shown to be relatively safe and possibly efficacious, with results of a large clinical trial available soon.
Aspirin. The value of aspirin in acute ischemic stroke has recently been assessed in two large trials involving nearly 40,000 participants.17,18 In these trials, aspirin (160 to 300 mg per day) was started, on average, between 12 and 24 hours after stroke onset.17,18 Based on outcome at six months, those who received early aspirin had a statistically significant reduction in the likelihood of death or severe disability, but the magnitude of the reduction was small: death or disability was reduced by about one case per 100 patients treated with early aspirin compared with delayed aspirin. Because aspirin or other antiplatelet agents are often used long term for secondary stroke prevention, it makes sense to begin aspirin therapy early (after the CT scan has excluded hemorrhage) to capture this modest benefit, in the absence of contraindications.
Heparin. Recent clinical trials have assessed the risks and benefits of heparin, heparinoids and low-molecular-weight heparins in patients with acute ischemic stroke. In the International Stroke Trial,17 participants were randomized to receive subcutaneous heparin in a dosage of 5,000 U twice daily, 12,500 U twice daily, or none. Fewer recurrent ischemic strokes occurred in those given heparin, but this improvement was offset by an increase in hemorrhagic strokes, for no net benefit. Critics suggest that dose-adjusted intravenous heparin might minimize hemorrhagic toxicity, but the fact remains that heparin has not been shown to improve neurologic outcome in adequate clinical trials. In the NINDS-sponsored Trial of ORG 10172 in Acute Stroke Treatment,19 administration of a heparinoid within 12 hours of acute ischemic stroke showed no clear benefit, with an increased risk of non–central nervous system bleeding. A trial of a low-molecular-weight heparin in acute ischemic stroke also showed no statistically significant difference in outcome at three months after stroke compared with placebo, but a difference favoring low-molecular-weight heparin appeared after six months.20 A large trial of this agent has just been completed in an effort to confirm this surprising benefit; the results showed no benefit in patients treated with low-molecular-weight heparin and an excess of intracerebral hemorrhage in the treated group.21
Cytoprotective Agents. These agents increase the tolerance of neurons to ischemia and have shown promising results in experimental models. None, however, have been shown to be beneficial in adequate clinical trials to date. Large trials testing citicoline, clomethiazole and glycine antagonist should be completed soon. These agents appear to be safer than fibrinolytic therapies and may have a longer time window for efficacy.
Final Comment
The management of acute ischemic stroke has entered a new era of urgency and activism, ushered in by the availability of t-PA and increasing appreciation of the importance of supportive care (Table 5). Patients at high risk for stroke must be taught to respond to early symptoms, and hospitals should organize clinical protocols and pathways for the effective implementation of stroke therapies.
| Determine whether stroke is ischemic or hemorrhagic by computed tomography | |
| Consider administration of t-PA if less than three hours from stroke onset | |
| General management: | |
| Blood pressure (avoid hypotension!) | |
| Assure adequate oxygenation | |
| Administer intravenous glucose | |
| Take dysphagia/aspiration precautions | |
| Consider prophylaxis for venous thrombosis if patient is unable to walk | |
| Suppress fever, if present | |
| Assess stroke mechanism (e.g., atrial fibrillation, hypertension) | |
| Consider aspirin therapy if ischemic stroke and no contraindications (not within 24 hours of t-PA) | |