
A more recent article on disorders of puberty is available.
Am Fam Physician. 1999;60(1):209-218
See related patient information handout on early and delayed puberty, written by the authors of this article.
Normal puberty begins between eight and 14 years of age in girls and between nine and 14 years of age in boys. Pubic hair distribution is used to stage puberty, along with breast size and contour in girls and testicular volume in boys. Some children experience constitutional sexual precocity, but precocity is likely to be pathologic if it occurs in very young children, if there is contrasexual development or if the sequence of normal pubertal milestones is disrupted. Delayed puberty may be constitutional, but pathologic causes should be considered. The etiology of a pubertal disorder can often be determined with the use of a focused medical history, a directed physical examination and appropriate diagnostic tests. Treatment for disorders of puberty is determined by the underlying cause.
Puberty is a process leading to physical and sexual maturation that involves the development of secondary sexual characteristics as well as growth, changes in body composition and psychosocial maturation.
Normal Development
Two processes contribute to the physical manifestations of puberty: adrenarche and gonadarche. Adrenarche normally occurs between six and eight years of age with increased adrenal androgen secretion; its exact biologic role is not well understood. It is accompanied by changes in pilosebaceous units, a transient growth spurt and the appearance of axillary and pubic hair in some children, but no sexual development.1 Gonadarche is initiated by the macroneurons of the hypothalamus that secrete gonadotropin-releasing hormone (GnRH) in a critical pulsatile pattern that regulates the release of follicle-stimulating hormone (FSH) and luteinizing hormone (LH) by the anterior pituitary.2 In boys, LH stimulates testosterone production by the Leydig cells and, after spermarche, FSH supports the maturation of spermatozoa. In girls, FSH stimulates estrogen production and follicle formation and, after ovulation, LH stimulates the development of the corpus luteum.
The standard clinical system for describing normal pubertal development and its variations is the five-stage system established by Tanner and Marshall (Tables 1 and 2).3–5 Girls begin puberty with breast buds and skeletal growth, followed by the arrival of pubic hair, axillary hair and menarche. Initially, boys have testicular enlargement followed by the appearance of pubic hair, enlargement of the penis and spermarche. Skeletal and muscle growth are late events in male puberty. The age at which pubertal milestones are attained varies among the population studied and is influenced by activity level and nutritional status. Girls with low body fat (e.g., competitive athletes) may have a significant delay in menarche (up to a year or more).6
| Tanner stage | Breasts* | Standard | Pubic hair* | Standard | Growth | Other |
|---|---|---|---|---|---|---|
| 1 | Prepubertal, elevation of papilla only | Prepubertal, villus hair only | — | Basal: about 5.0 to 6.0 cm (2.0 to 2.4 in) per year | Adrenarche Ovarian growth | |
| 2 | Breast bud appears under enlarged areola (11.2 years) | Sparse growth of slightly pigmented hair along the labia (11.9 years) | Accelerated: about 7.0 to 8.0 cm (2.8 to 3.2 in) per year | Clitoral enlargement Labia pigmentation Uterus enlargement | ||
| 3 | Breast tissue grows beyond areola without contour separation (12.4 years) | Hair is coarser, curled and pigmented; spreads across the pubes (12.7 years) | Peak velocity: about 8.0 cm (3.2 in) per year (12.5 years) | Axillary hair (13.1 years) Acne (13.2 years) | ||
| 4 | Projection of areola and papilla forms a secondary mound (13.1 years) | Adult-type hair but no spread to medial thigh (13.4 years) | Deceleration: < 7.0 cm (2.8 in) per year | Menarche (13.3 years) Regular menses (13.9 years) | ||
| 5 | Adult breast contour with projection of papilla only (14.5 years) | Adult-type hair with spread to medial thigh but not up linea alba (14.6 years) | Cessation at about 16 years | Adult genitalia |
| Tanner stage | Standard | Genitalia* | Pubic hair* | Growth | Other |
|---|---|---|---|---|---|
| 1 | Prepubertal Testes: < 2.5 cm (1.0 in) | Prepubertal, villus hair only | Basal: about 5.0 to 6.0 cm (2.0 to 2.4 in) per year | Adrenarche | |
| 2 | Thinning and reddening of scrotum (11.9 years) Testes: 2.5 to 3.2 cm (1.0 to 1.28 in) | Sparse growth of slightly pigmented hair at base of penis (12.3 years) | Basal: about 5.0 to 6.0 cm (2.0 to 2.4 in) per year | Decrease in total body fat | |
| 3 | Growth of penis, especially length (13.2 years) Testes: 3.3 to 4.0 cm (1.32 to 1.6 in) | Thicker, curlier hair spreads to the mons pubis (13.9 years) | Accelerated: about 7.0 to 8.0 cm (2.8 to 3.2 in) per year | Gynecomastia (13.2 years) Voice break (13.5 years) Muscle mass increase | |
| 4 | Growth of penis and glands, darkening of scrotum (14.3 years) Testes: 4.1 to 4.5 cm (1.64 to 1.8 in) | Adult-type hair but no spread to medial thigh (14.7 years) | Peak velocity: about 10.0 cm (4.0 in) per year (13.8 years) | Axillary hair (14.0 years) Voice change (14.1 years) Acne (14.3 years) | |
| 5 | Adult genitalia (15.1 years) Testes: > 4.5 cm (1.8 in) | Adult-type hair with spread to medial thighs but not up linea alba (15.3 years) | Deceleration and cessation (about 17 years) | Facial hair (14.9 years) Muscle mass continues to increase after Stage 5 |
Evaluation of Abnormal Puberty
The goal of the initial assessment of children with abnormal puberty is to distinguish children with benign constitutional causes for the abnormality from those with pathologic causes. A focused medical history, a directed physical examination, assessment using a complete growth chart and a radiograph of the left wrist to establish bone age can often help the physician make this distinction.
The history should focus on the child's previous growth and development and the precise timing and sequence of the physical milestones and behavior changes of puberty. A history of medical or surgical treatment may provide clues to an underlying pathologic condition. A detailed dietary history should be obtained in underweight children with delayed puberty. The family history may reveal important information about a familial pattern of precocious or delayed puberty, as well as evidence of genetic disease.
The physical examination should focus on the neurologic and endocrine systems. Examining the optic fundi, estimating the visual fields and evaluating the sense of smell can be helpful, but examination of the genitalia and determination of the status of the pubertal milestones is essential and should be documented for future reference. Photographs are well suited to this purpose. The detailed growth chart that begins at birth is used to estimate the annual growth rate (centimeters [inches] per year) and to determine if and when a growth spurt has occurred (an abrupt increase in the annual growth rate). A radiograph of the left wrist is used to estimate physiologic age for comparison with the child's chronologic age.
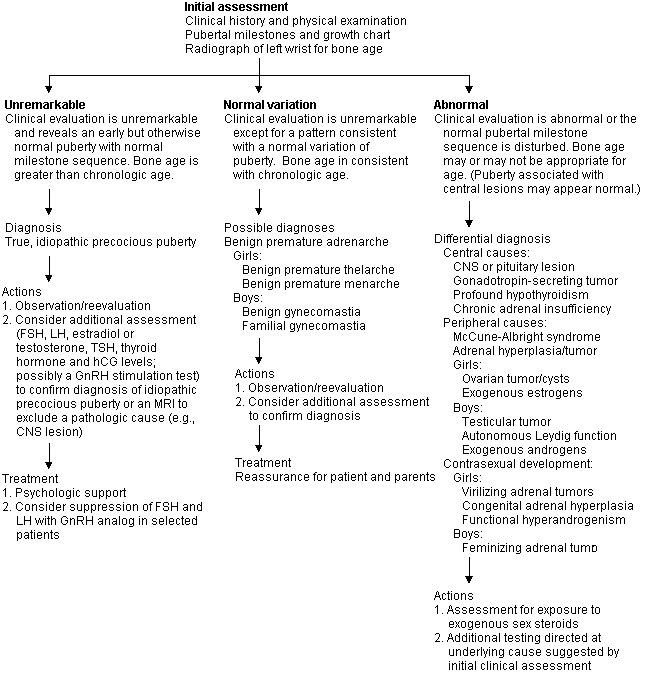
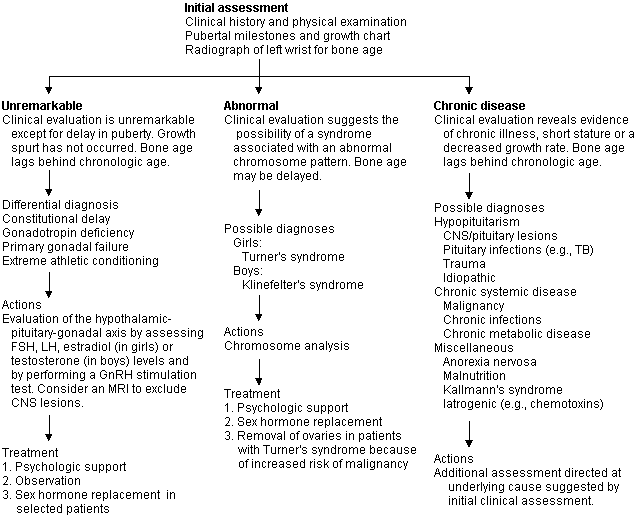
Premature and Atypical Puberty
If the initial assessment is unremarkable, the patient is likely to have a constitutional, central cause for premature puberty. Sometimes pathologic central causes produce premature puberty with normal sequences of milestones, giving the impression of benign causes, and variations of normal puberty may mimic pathologic puberty. Pathologic causes are likely if sexual development occurs in very young children or if there is contrasexual development (e.g., virilization of girls, feminization of boys). Peripheral causes are always pathologic and tend to produce an atypical puberty with loss of synchronicity of pubertal milestones (e.g., penile enlargement without testicular enlargement, extensive pubic hair, or menarche in the absence of breast buds). Girls have a benign central cause for precocious puberty about 50 to 90 percent of the time, but about one half of all boys with early puberty have a pathologic peripheral cause. Thus, all boys with precocious puberty should undergo detailed investigation, but in girls additional investigation can be based on the clinical impression.7
EVALUATION
Further diagnostic testing is used to confirm the initial impression of idiopathic precocious puberty, to localize the abnormality of the pathologic cause or to determine which imaging study to obtain. Tests for this purpose include serum levels of FSH and LH, estradiol, testosterone, thyroid-stimulating hormone (TSH), thyroxine (T4) and human chorionic gonadotropin (hCG). A cost comparison is summarized in Table 3. If a central pathologic cause is suspected after hormone measurements are obtained, magnetic resonance imaging (MRI) of the brain and pituitary gland, a GnRH stimulation test, or both, may be indicated. The GnRH stimulation test is performed by administering 100 μg of GnRH either intravenously or subcutaneously after overnight fasting.8 Serum levels of FSH and LH are measured at baseline just before the injection and at 15, 30, 45 and 60 minutes after the injection. Test interpretation is controversial, and the pattern of increases of FSH and LH vary with the stage of puberty, but if the patient has central precocious puberty, the hypothalamic-pituitary axis will have been activated, and a two- to threefold rise in FSH and LH will be observed.
| Test/profile | Cost* |
|---|---|
| FSH/LH | $ 57 |
| Estradiol | 46 |
| Testosterone | 58 |
| Prolactin | 48 |
| hCG, beta subunit (quantitative) | 44 |
| Thyroid panel (TSH, T4, T3, uptake) | 57 |
| GnRH | 322 |
If a peripheral cause is suspected from the initial clinical assessment or by the pattern of hormone levels, high-resolution ultrasonographic images of the ovaries or computed tomographic images of the adrenal glands are indicated, depending on the clinical impression. Measurement of serum levels of 17-hydroxyprogesterone and dehydroepiandrosterone (DHEA) is advised, especially in girls with virilization.
DIFFERENTIAL DIAGNOSIS
Some children cannot be easily placed in a diagnostic category even after extensive evaluation. When treatment is necessary, it is directed at the underlying cause. Parental and patient anxiety is common when sexual development is abnormal. Such anxiety should be managed with reassurance and a supportive demeanor. Children with premature sexual development are at risk for psychopathology9 and sexual abuse,10 and may benefit from psychotherapy.
Benign Premature Adrenarche. This is a self-limited condition occurring before six years of age that is characterized by the appearance of pubic and, occasionally, axillary hair, increased sebaceous activity and adult-type body odor but no sexual development. These children have statistically normal growth patterns and chronologically appropriate bone age but may be tall compared with other family members. A modest elevation of serum DHEA to the range found normally in early puberty is characteristic.11 However, other adrenal steroid hormone levels are normal, and sex hormone levels are in the prepubertal range. An adrenocorticotropic (ACTH) stimulation test is used to exclude late-onset congenital adrenal hyperplasia.12 The GnRH test demonstrates a prepubertal pattern, and imaging studies are normal. No treatment other than reassurance is required.
Benign Premature Thelarche. This condition can occur in girls as young as 18 months and is characterized by premature limited breast development without progression to a mature breast contour. Other signs of puberty do not occur, and the growth rate is prepubertal, with a bone age appropriate for chronologic age. Levels of gonadotropins and estradiol are normal, and ultrasound images of the ovaries are unremarkable. The condition usually resolves spontaneously and requires no treatment. A biopsy of the breast tissue should not be obtained because it is the anatomic equivalent of a partial mastectomy, and subsequent breast development will be altered.
Benign Premature Menarche. This is a rare and poorly understood disorder similar to benign premature thelarche and is thought to be due to transient ovarian activity that is self-limited. Some cases may be attributed to exposure to exogenous estrogens of either a medical (such as oral contraceptives) or an agricultural origin.13 Observation and repeated evaluation constitute an appropriate strategy unless another diagnosis is suspected.
Benign Gynecomastia of Adolescence. This is a nearly universal finding among boys in middle to late puberty.14 The breast tissue is usually asymmetric and often tender. If the history and physical examination, including palpation of the testicles, are unremarkable, reassurance and periodic reevaluation are all that is necessary. Most cases resolve in one to two years.
Familial gynecomastia is a fairly common heterogenous disorder transmitted as an X-linked recessive trait or a sex-limited dominant trait causing limited breast development around the time of puberty. It requires no further evaluation in an otherwise normal boy unless associated with hypogonadism.15 Those with severe gynecomastia may require cosmetic surgery.
Pathologic gynecomastia occurs in cases of Klinefelter's syndrome and prolactin-secreting adenomata, and in response to a wide variety of drugs (e.g., marijuana, phenothiazines).
Constitutional and Idiopathic Precocious Puberty. Children with these conditions have premature but otherwise normal-appearing pubertal development. Girls develop breasts and have an early growth spurt with a radiographic bone age in excess of their chronologic age. This causes them to be taller than their peers, but epiphyseal closure occurs early and they mature into short adults. Boys have testicles that are enlarged symmetrically without masses. These children have pubertal levels of FSH, LH and sex hormones, and a pubertal response to the GnRH stimulation test. Thyroid and adrenal hormone levels are normal, as are diagnostic images of the brain, pituitary gland, adrenals and ovaries.
The goal of medical treatment is to return the patient to a prepubertal pattern of growth and development, which is accomplished with the use of one of the GnRH analogs, such as long-acting injectable leuprolide (Lupron) or short-acting intranasal nafarelin (Synarel). These agents desensitize the anterior pituitary gland to the normal pulsatile stimulation of hypothalamic GnRH. Release of FSH and LH by the anterior pituitary may increase initially but, over time (up to three months), release of FSH and LH is suppressed.16 In turn, production of sex hormones declines. Although individual responses vary, therapy offers the greatest advantage for children in whom puberty begins at a very early age, those who demonstrate rapidly accelerating bone age, and those with lower genetic height potential.17
Central Nervous System and Pituitary Lesions. These conditions may cause a clinical picture similar to idiopathic precocious puberty but may be associated with neurologic problems (e.g., visual field defects). Newer, high-resolution MRI techniques may be identifying pituitary or central nervous system lesions that were previously undetectable, resulting in a decline in “idiopathic” cases. Treatment varies with the type of lesion involved.
Gonadotropin-Secreting Tumors. These tumors are uncommon but typically secrete the 3 subunit of hCG or, in rare cases, the β subunit. In boys, this produces a clinical syndrome of incomplete precocious puberty. Girls do not demonstrate premature sexual development from hCG alone; FSH priming is required for estradiol production by the ovaries. Tumors that secrete hCG include hepatomas or hepatoblastomas; teratomas or chorioepitheliomas of the gonads, mediastinum, retroperitoneum or pineal gland; and germinomas of the pineal gland.9 FSH-and LH-secreting tumors are rare. The treatment is usually surgical.
Peripheral Precocious Puberty. This disorder usually produces a clinical picture of incomplete puberty in which some of the milestones of puberty do not appear or fail to achieve the usual synchronicity. Development occurs despite low or prepubertal levels of FSH and LH. Ovarian or adrenal androgens may produce virilization in girls. Clinical investigation may identify the type and source of sex hormone stimulation (Figure 1). Treatment depends on the etiology.
McCune-Albright Syndrome. Patients with this disorder present with the classic triad of polyostotic fibrous dysplasia, café au lait spots and precocious puberty.18 It appears to be a heterogeneous syndrome with multiple types of inheritance patterns and may be associated with hyperthyroidism or Cushing's syndrome. Treatment includes medical and orthopedic management.
Exogenous Sex Hormones. Ingestion of sex hormones by prepubertal children causes the development of secondary sexual features in conjunction with suppressed FSH and LH levels. Girls who take estrogens (oral contraceptives) may develop dark-brown breast areolae that are not usually associated with endogenous types of precocious puberty, and those who have not begun natural puberty will lack pubic hair. Boys taking androgens will have prepubertal-sized testicles. In one survey, 7 percent of male high school seniors were found to have used anabolic steroids, most before 16 years of age.19 Metabolites of the exogenous hormones may be recovered from the urine.
Contrasexual Development. Some form of hyperandrogenism, functional or pathologic, must be considered in any girl with premature or excessive pubic hair. The most common causes are nonclassic adrenal hyperplasia, premature or exaggerated adrenarche in peripubertal girls, and polycystic ovaries in older girls. Hirsutism may be idiopathic or familial, but it also may be produced by some oral contraceptives.20 However, when signs of virilization (e.g., clitoromegaly, increased muscle mass) accompany signs of defeminization (e.g., loss of female contours and breast mass), the cause is always pathologic, and ovarian or adrenal tumors, Cushing's syndrome, hyperprolactinemia, acromegaly, exogenous androgens or abnormalities of androgen action or metabolism must be considered.21 In boys, contrasexual development may be caused by rare, estrogen-secreting adrenal tumors.
Delayed Sexual Development
A diagnosis should be sought for all children with delayed sexual development, which is usually associated with short stature. In girls, delayed sexual development is defined as lack of any breast development by 14 years of age or when more than five years pass between initial growth of breast tissue and menarche. Girls who develop secondary sexual characteristics but fail to achieve menarche by 16 years of age should be evaluated for primary amenorrhea. In boys, delayed sexual development is defined as no testicular enlargement by 14 years of age or the passing of five years between the initial and complete development of the genitalia. Patients with delayed puberty may be classified into one of three groups (Figure 2), based on the initial clinical assessment: (1) those who appear otherwise normal; (2) those who have the stigma of a chromosomal abnormality; and (3) those who appear to have some type of chronic disease.
EVALUATION
Additional testing will be determined by the initial clinical assessment and suspected etiology. Routine diagnostic laboratory profiles might yield important information if an ill-defined chronic disease is suspected. If a hormone abnormality is suspected, determination of the serum levels of TSH, T4, FSH, LH and prolactin is usually indicated, and a GnRH stimulation test will be useful. MRI scanning of the brain and pituitary gland is indicated if an abnormality of the hypothalamic-pituitary axis is suspected. Since not every adolescent with chromosomal abnormalities will have the classic clinical features, a chromosome analysis should be considered, especially in short girls with delayed puberty.
DIFFERENTIAL DIAGNOSIS
The differential diagnosis of delayed puberty is extensive, but most children who do not have an underlying chronic disease will be found to have a constitutional delay, some form of hypopituitarism, or gonadal failure associated with a sex chromosome abnormality.
Constitutional Delay. Puberty can be delayed in otherwise healthy children. Typically these children have a normal length and weight at birth and appear to grow normally for a few years but then fall below the fifth percentile on standard growth curves, at which time growth velocity returns to a normal rate and continues along a low percentile curve. They demonstrate prepubertal levels of FSH, LH and estradiol or testosterone. Since the GnRH stimulation test shows a prepubertal response, it is difficult to distinguish this condition from a pathologic gonadotropin deficiency unless a structural or biochemical abnormality of the hypothalamic-pituitary axis is found. Spontaneous puberty occurs, allowing the child to become a normal adult. Because of this clinical course, treatment is controversial.
Some authorities recommend continued observation as the only therapy, while others recommend induction of puberty with sex steroids.22 Puberty may also be delayed in children who are elite athletes involved in extensive training, those who have an eating disorder and those who are malnourished.
Hypopituitarism. This condition is caused by a variety of diseases that affect the hypothalamic-pituitary axis.23 Depending on the endocrine functions affected, children may present with growth failure, secondary hypothyroidism, adrenal insufficiency and diabetes insipidus, as well as delayed puberty. Kallmann's syndrome is associated with anosmia or hyposmia, and hypogonadotropic hypogonadism. Treatment is directed at the underlying cause and includes initiation of hormone replacement therapy.
Chromosomal Abnormalities. These abnormalities may be associated with delayed puberty. In girls, the most common is Turner's syndrome (about one case in 3,000 live female births). In these cases, patients may present with only growth failure and pubertal delay, or with the more pathognomonic features of this disorder: inner canthal folds with ptosis, a short webbed neck and a “shield chest” with widely spaced nipples.24 In boys, the most common abnormality is Klinefelter's syndrome (about one case in 700 live male births); patients typically are tall, with a eunuchoid body (i.e., long legs and relatively short arms, a height:arm-span ratio greater than 1.0). The testes are small but firm, and gynecomastia is often present.25 Other syndromes (e.g., Noonan's syndrome) can also be associated with delayed puberty.
Final Comment
Many physical and biochemical problems associated with disorders of puberty may be successfully treated. Puberty is an arduous process for adolescents when normal, but it is more difficult in children with aberrant puberty. These children benefit from management by a knowledgeable and sensitive clinician. Psychotherapy can play an important role in assisting these patients as they develop physically and emotionally.