
A more recent article on polycystic ovary syndrome is available.
Am Fam Physician. 2003;68(4):697-705
Polycystic ovary syndrome has been viewed primarily as a gynecologic disorder requiring medical intervention to control irregular bleeding, relieve chronic anovulation, and facilitate pregnancy. A large body of evidence has demonstrated an association between insulin resistance and polycystic ovary syndrome. The former condition has an established link with long-term macrovascular diseases such as type 2 diabetes mellitus, hypertension, and atherosclerotic heart disease, consequences that also are observed in women with polycystic ovary syndrome. In addition, chronic anovulation predisposes women to endometrial hyperplasia and carcinoma. The purpose of this review is to examine the clinical course of this syndrome, which spans adolescence through menopause, and suggest a simple and cost-effective diagnostic evaluation to screen the large numbers of women who may be affected. Therapy, which should be individualized, should incorporate steroid hormones, antiandrogens, and insulin-sensitizing agents. Weight loss by way of reduced carbohydrate intake and gentle exercise is the most important intervention; this step alone can restore menstrual cyclicity and fertility, and provide long-term prevention against diabetes and heart disease. Treatment alternatives should be directed initially toward the most compelling symptom. Longitudinal care is of paramount importance to provide protection from long-term sequelae.
Polycystic ovary syndrome (PCOS) is the most common endocrinopathy among women of reproductive age and is estimated to affect up to 10 percent of the U.S. population or approximately 5 million women.1 In 1935, Stein and Leventhal2 described masculinized women with amenorrhea, sterility, and enlarged ovaries containing multiple cysts. The syndrome was placed in the gynecologic realm for control of chronic anovulation, abnormal menstrual bleeding, and infertility.
By the early 1980s, this symptom complex had been linked to hyperinsulinemia and impaired glucose tolerance.3,4 The connection to an insulin post-receptor defect was isolated in women with PCOS in the early 1990s.5 As a result of these recent associations, attention is now focused on treating the central deficits and fundamental problems of hyperandrogenism, hyperinsulinemia, abnormal serum lipid levels, and obesity that have broader health implications (Table 1).3,6–10 This new information profoundly alters our view of the gravity of this condition. Family physicians are well placed to make early diagnoses of PCOS and to help patients avoid the long-term consequences.
| Infertility | |
| Recurrent spontaneous abortion | |
| Depression/anxiety | |
| Dyslipidemias | |
| Total cholesterol (elevated) | |
| LDL cholesterol (elevated) | |
| HDL2 cholesterol (decreased) | |
| Triglycerides (elevated) | |
| Hypertension | |
| Type 2 diabetes mellitus | |
| Coronary atherosclerosis | |
| Cerebrovascular accidents | |
| Endometrial carcinoma | |
Clinical Course
Young women of reproductive age most frequently seek attention initially because of irregular menses, hirsutism, or infertility, but PCOS has a long prodrome with detectable abnormalities throughout the life cycle of affected women. The earliest manifestations of PCOS are discernible in the peripubertal years.
Ovarian hyperandrogenism and insulin resistance develop with increased frequency in adolescent girls who have premature pubarche.11,12 In the early reproductive period, chronic anovulation results in reduced rates of conception. When pregnancy is achieved, it frequently terminates in spontaneous, first-trimester loss or is associated with gestational diabetes.6 Approximately 25 to 30 percent of these women show impaired glucose tolerance by the age of 30, and 8 percent of women with PCOS develop frank type 2 diabetes mellitus annually.7
Markers of premature coronary artery and cerebrovascular disease are prevalent. Women with polycystic ovaries are seen to have more extensive coronary artery disease by angiography.8 In two case-control studies,9,10 women in their 40s had greater intima-medial thickness of the carotid vessels, and more atherogenic lipid profiles: increased total and low-density lipoprotein (LDL) cholesterol and triglyceride levels, and decreased high-density lipoprotein (HDL) cholesterol levels.9,10
These metabolic abnormalities are compounded by the prevalence of obesity, which occurs in more than 65 percent of women with PCOS.3 Abnormal androgen production declines as menopause approaches (as it does in women without PCOS), and menstrual patterns somewhat normalize. However, in retrospective cohort studies,13,14 perimenopausal and postmenopausal women with a history of PCOS had increased rates of type 2 diabetes, hypertension, and coronary artery disease compared with control patients. PCOS appears to follow a familial distribution; 40 percent of the sisters and 20 percent of the mothers of affected women also have the syndrome to varying degrees.15
Clinical Features
In many women the symptoms are easily recognizable, but ethnicity influences the extent of symptoms, especially with regard to hirsutism and obesity. Therefore, taking a diligent history with regard to menstrual patterns is crucial to help establish the diagnosis. The National Institute of Child Health and Development16 held a consensus meeting to develop the following diagnostic criteria for PCOS: (1) clinical or biochemical evidence of hyperandrogenism; (2) oligo-ovulation; and (3) exclusion of other known disorders, such as congenital adrenal hyperplasia or hyperprolactinemia.
HYPERANDROGENISM
The wide spectrum of manifestations ranges from mild acne and increased terminal (coarse) hair growth in midline structures (face, neck, abdomen), to android changes in body habitus, with waist-to-hip ratios of more than 1. Variations are influenced by ethnicity,17 as well as coexisting conditions (such as hyperthyroidism) that alter androgen biosynthesis. For example, Asian women with PCOS are rarely hirsute, but hirsutism is a frequent finding in black women with PCOS. Yet the actual incidences of hyperandrogenemia and insulin resistance do not show a racial predilection.
In addition, nonhirsute women with oligo-ovulation may have laboratory evidence of hyperandrogenism. Frank or rapid “virilization” involving clitoromegaly, vocal chord thickening, or male-pattern baldness is rare in patients with PCOS and, when present, suggests another cause of hyperandrogenism, such as adrenal disorders or androgen-producing tumors (Table 2).18
OLIGO-OVULATION
Oligo-ovulation manifests as menstrual irregularity and occurs in 70 percent of women with PCOS. Among women with more regular menses, many have variable degrees of ovulatory dysfunction. Often the menstrual formula (i.e., three to five days of menstrual flow every 28 to 35 days) occurs for the first one to two years after menarche (which occurs at the normal age), but menses then become less frequent, occurring every 45 to 365 days. Because the estrogen from ovarian and adipose tissues stimulates proliferation of endometrium that is not stabilized by post-ovulatory progesterone, bleeding can be unpredictable, heavy, and prolonged. Chronic endometrial proliferation can result in carcinoma.
SYMPTOMS WITH VARIABLE FREQUENCY
Obesity
More than 65 percent of women with PCOS have a body mass index exceeding 27. The fat distribution often is abdominal/visceral, similar to that frequently associated with metabolic abnormalities (e.g., hypertension, dyslipidemia, insulin resistance, glucose intolerance). Most women deny childhood obesity and describe normal weight until after menarche. Significant weight gain appears in the mid-teens and accelerates in the later teens and early 20s.
The presence of obesity also is influenced by ethnicity. It is most common in Hispanic, black, and white women, less striking in women of Mediterranean descent, and rare in Asian women.17 Obesity is likely to facilitate the metabolic abnormalities of PCOS, as evidenced by the reduction in insulin resistance and restoration of cyclic menses following weight loss.19 A 1982 study,20 which has been confirmed by later research, showed that a 10 to 15 percent weight reduction resulted in spontaneous conception in more than 75 percent of obese patients with PCOS.
Acanthosis Nigricans
These velvety, raised skin deposits in intertriginous areas are associated with insulin resistance and result from insulin stimulation of the basal layers of the epidermis. When found in conjunction with hyperandrogenism, the condition is termed HAIR-AN syndrome (hyperandrogenic-insulin resistant–acanthosis nigricans); it occurs in 2 to 5 percent of hirsute women.21 The majority of women with PCOS (70 percent) are insulin resistant, but hyperinsulinemia is far more severe in women with HAIR-AN syndrome.
Polycystic Ovaries
Ovaries with multiple, small (less than 10 mm) follicular cysts surrounding the ovarian stroma are found in 16 to 25 percent of normal women and in female patients with ammenorrhea caused by other etiologies.22 Nearly 80 percent of women with hyperandrogenism have polycystic ovaries,23 but these may not be present at the time of evaluation in women who have used oral contraceptive pills (OCPs), insulin-sensitizing agents, or other forms of ovarian suppression. Therefore, the presence of polycystic ovaries on ultrasonography is not a diagnostic essential.
Diagnostic Evaluation
Although there is no consensus as to which laboratory tests should be used to diagnose PCOS, most physicians agree that the evaluation should screen for hyperandrogenemia as well as for abnormalities that have serious health consequences. Often, the clinical picture is readily apparent from the history and physical findings. Thus, testing for parameters known to be abnormal in women with PCOS, such as luteinizing hormone (LH) and follicle-stimulating hormone (FSH) ratios, is unnecessary, redundant, and expensive.
In the author's opinion, the evaluation should follow these principles: exclude other etiologies of amenorrhea, such as prolactin or thyroid abnormalities; exclude other causes of hyperandrogenism; exclude glucose intolerance; and detect insulin resistance and lipid abnormalities.
LABORATORY EVALUATION
Normal testosterone determinations in the hirsute patient can be misleading, partially because of inherent variation in commercial testing methods.24 However, bioavailable (free) testosterone levels may support the diagnosis, especially in a nonhirsute woman with other signs and symptoms of PCOS. Hyperandrogenemia also might be established by elevated serum levels of dehydroepiandrosterone sulfate (DHEAS) and androstenedione.4
Both free testosterone and sex hormone-binding globulin may be useful in monitoring the efficacy of androgen suppression therapy. Total testosterone levels greater than 20 ng per dL (0.7 nmol per L) or DHEAS levels greater than 700 ng per mL (1.9 μ mol per L) are suggestive of androgen-secreting tumors; these patients should be referred for gynecologic investigation.25
Morning 17α-hydroxyprogesterone (17-OHP) determination is important to exclude nonclassic adrenal hyperplasia secondary to 21-hydroxylase deficiency, which is present in 1 to 8 percent of hirsute women.26 When elevated 17-OHP is discovered, the next step should be a short adrenocorticotropic hormone stimulation test to further delineate the enzymatic defect. The ratio of LH to FSH is greater than 3:1 in about 30 percent of women with PCOS and may be diagnostically helpful in nonhirsute women with mild ovulatory dysfunction, but this determination is not routinely necessary if the clinical picture is otherwise clear.
Because nearly 30 percent of women with PCOS have impaired glucose tolerance, determinations of glucose tolerance and insulin resistance are of paramount importance. One approach is to perform standard oral glucose tolerance testing with insulin levels. Peak levels of insulin that exceed 100 μ U per mL (718 pmol per L) are suggestive of insulin resistance. This test may be unnecessarily cumbersome and expensive for general screening, however, and the author's practice is to reserve this test for use in women with a family history of glucose intolerance, morbid obesity, or other symptoms suggestive of diabetes.
A more efficient assessment may be to use the ratio of fasting levels of glucose to insulin. When less than 4.5, this ratio has a significant correlation with insulin resistance and has been studied for use as a screening test in obese patients with PCOS. It combines sensitivity (95 percent) and specificity (84 percent) for insulin resistance, with positive and negative predictive values of 87 percent and 97 percent in obese patients with PCOS.27 This predictive capacity may not hold true in nonobese women with PCOS.
The author includes assessments of total, HDL, and LDL cholesterol levels as well as triglyceride levels to h elp in planning and follow-up of recommended dietary modifications to reduce obesity and cardiovascular risk. Finally, endometrial biopsy is helpful to rule out endometrial hyperplasia in patients with prolonged amenorrhea (more than five months). A list of laboratory tests that may help to identify patients with PCOS is provided in Table 3.
| Test | Normal value | Purpose |
|---|---|---|
| β-hCG | < 5 mIU per mL (< 5 IU per L) | Exclude pregnancy |
| TSH | 0.5 to 4.5 μU per mL (0.5 to 4.5 mU per L) | Exclude thyroid dysfunction |
| Prolactin | < 20 ng per mL (< 20 μ g per L) | Exclude hyperprolactinemia |
| Testosterone (total) | < 20 ng per dL (< 0.7 nmol per L) | Exclude androgen-secreting neoplasm |
| Testosterone (free) | 20 to 30 years—0.06 to 2.57 pg per mL (0.20 to 8.90 pmol per L) | Establish diagnosis or monitor therapy |
| 40 to 59 years—0.4 to 2.03 pg per mL (1.40 to 7.00 pmol per L) | ||
| DHEAS | 600 to 3,400 ng per mL (1.6 to 9.2 μmol per L) | Exclude androgen-secreting neoplasm |
| Androstenedione | 0.4 to 2.7 ng per mL (1.4 to 9.4 nmol per L) | Establish diagnosis |
| 17 α-hydroxyprogesterone | Follicular phase < 2 μg per L (6.1 nmol per L) | Exclude NCAH |
| Fasting insulin | < 20 μU per mL (< 144 pmol per L) | Exclude hyperinsulinemia |
| Fasting glucose | 65 to 119 mg per dL (3.6 to 6.6 mmol per L) | Exclude type 2 diabetes or glucose intolerance |
| Fasting glucose: insulin ratio | @ 4.5 | Exclude insulin resistance |
| Cholesterol (total) | 150 to 200 mg per dL (1.5 to 2 g per L) | Monitor lifestyle changes |
| HDL cholesterol | 35 to 85 mg per dL (0.9 to 2.2 mmol per L) | Monitor lifestyle changes |
| LDL cholesterol | 80 to 130 mg per dL (2.1 to 3.4 mmol per L) | Monitor lifestyle changes |
| Pelvic ultrasonography | Monitor lifestyle changes | |
| Endometrial biopsy | Negative for hyperplasia/malignancy | Exclude malignancy or hyperplasia |
Treatment
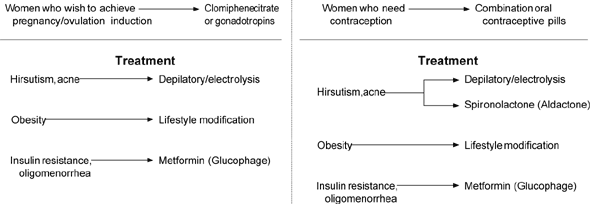
The primary goal of all forms of therapy is to suppress insulin-facilitated, LH-driven androgen production. Although numerous medications (Table 4) and protocols are effective in reducing insulin and androgen levels, the choices should be based on the woman's most pressing concern and her stage of reproductive life (Figure 1). Given the current awareness of the long-term consequences associated with PCOS, the physician carries the responsibility of tailoring therapy to add preventive benefits. In addition, cosmetic alterations can result in depression and withdrawal from social and career pursuits. Thus, the physician should be armed with information about removal of unwanted hair. Shaving and depilation are the most efficient short-term means for terminal hair removal while initiating androgen suppression using some of the modalities described below.
| Drug | Suggested dosage | Benefits* | Cost (generic)† |
|---|---|---|---|
| Oral contraceptives | 21 days per month | 1, 2, 3 | $27.00 to 32.00 |
| Medroxyprogesterone acetate (Provera) | 10 mg daily for 10 days | 2 | 12.00 (5.00 to 7.00) |
| Micronized progesterone (Prometrium) | 400 mg daily for 10 days | 2 | 30.00 |
| Spironolactone (Aldactone) | 50 to 200 mg daily | 1, 2‡ | 30.00 to 99.00 (25.00 to 85.00) |
| Metformin (Glucophage) | 500 to 850 mg three times daily | 1, 2, 3, 4, 5, 6 | 70.00 to 120.00 (63.00 to 108.00) |
| Clomiphene citrate (Serophene) | 50 to 150 mg for five days | 2, 3, 4 | 49.00 to 147.00 (29.00 to 103.80) |
| Gonadotropin (FSH/LH) | Individualized‡ | 2, 3, 4 | 600.00 to 3,000.00 |
STEROID HORMONES
OCPs are the most efficient means of androgen suppression (ovarian as well as adrenal), and nearly any combination OCP is effective in treating PCOS. This therapy results in lower and static LH levels without surges. The estrogen component stimulates hepatic production of sex hormone-binding globulin that reduces bioavailable androgen and can reduce hirsutism and acne. The progestin component provides competitive antagonism to androgen at its receptors, reducing the action of testosterone at the target organ.
Newer formulations contain progestins of lower androgenicity, such as norgestimate, desogestrel, and gestodene. OCPs can be used alone or in conjunction with antiandrogens, gonadotropin-releasing hormone agonists, or insulin-sensitizing agents. Restoration of the menstrual cycle and prevention of endometrial hyperplasia are additional benefits.
Hirsute women should be counseled that regression of terminal hair growth during OCP therapy is a slow process, requiring up to eight months for noticeable benefits. It is appropriate to institute permanent methods of hair removal by way of electrolysis or laser ablation after suppression of hyperandrogenism has been achieved.
Because these women are highly estrogenized (as a result of peripheral conversion of androgenic precursors), menses can be induced by administration of progestational agents for approximately 10 days. Women should be advised that their cycles are not re-established by this treatment, and that if it is not repeated at two- or three-month intervals, unchecked endometrial growth can result in endometrial hyperplasia. If menstrual bleeding does not occur within 14 days of completion of progesterone administration, another etiology for amenorrhea rather than PCOS should be sought. Progesterone administration does not provide relief from any other symptoms or prevent any of the long-term conditions. It is useful on a scheduled basis only for menstrual regulation.
ANTIANDROGENS
Therapy with spironolactone (Aldactone) has a direct suppressive effect on enzymes in the androgen biosynthetic pathway. Because of its structural similarity to testosterone, it can competitively inhibit androgen receptor binding and is useful in the treatment of hirsutism. Three of six open clinical trials showed improvement in hirsutism, but results of double-blind placebo-controlled trials were not as positive.28 One study29 has shown that spironolactone can reduce the caliber and growth rate of terminal hair. Dosages as high as 200 mg per day are tolerated, and menses sometimes resume. This agent is most effective when combined with OCPs; some form of contraception is advisable when it is used alone. A newly released formulation of OCP contains an analog of spironolactone, drospirenone. Drospirenone combined with ethinyl estradiol (Yasmin) is indicated as a contraceptive.
ASSISTED FERTILITY
Clomiphene citrate (Serophene) or gonadotropins can be used to establish menses but should be reserved for use in patients who wish to become pregnant. Because the woman with PCOS is uniquely sensitive to these agents and risks higher rates of ovarian hyperstimulation and multiple gestation,30 specialty referral for this therapy is recommended. Clomiphene administration will result in ovulation in 50 to 60 percent of cases, in pregnancy in 30 percent, and in multiple gestation (twins or greater) in 3 percent.
Although the body of data is limited, it is suggested that excessive or prolonged exposure to clomiphene citrate (more than 12 months) could increase the risk of ovarian cancer.31 When clomiphene fails, gonadotropin administration frequently is successful in helping women to achieve pregnancy (70 percent), but it requires self-injection, is costly, and carries an even higher rate of hyperstimulation and multifetal pregnancy (as high as 30 percent).32
INSULIN REDUCTION
Metformin (Glucophage), a biguanide and insulin-sensitizing agent, has been used to restore menstrual cyclicity and induce ovulation in PCOS without the use of additional fertility drugs. Use of this agent is associated with reductions in serum levels of bioavailable androgen, LH, and atherogenic lipids. Levels of plasminogen activator inhibitor I and Lp(a) lipoprotein also were reduced.33 There are no data to show a benefit in women who are not insulin resistant, but a few physicians support its empiric use.
Metformin is presently classified as a category “B” risk in pregnancy, which indicates that there are no apparent fetal defects associated with its use in the late trimester of pregnancy, based on animal studies. Because the human effects of first-trimester use are unknown, discontinuation of therapy at the onset of pregnancy confirmation has been standard. However, a recent prospective study of women with PCOS who continued metformin therapy through the first trimester of pregnancy showed no evidence of fetal harm, calling into question this recommendation.34
The rate of early spontaneous abortion was reduced from 73 percent without metformin to 10 percent. In our clinical setting, the initial dosage of 500 mg is given at bedtime each night for one week to reduce the occurrence of gastrointestinal side effects and is increased by 500 mg per week to a total dosage of 1,500 mg per day in divided doses. Insulin, glucose, and androgen levels are retested after eight weeks. If improvement is not shown, the dosage is increased to 2,550 mg. Menses often will occur within this treatment time. If fertility is desired, ovulation induction agents can be added. Metformin therapy is associated with a slight risk of lactic acidosis and is not used in patients with impaired liver or renal function.
LIFESTYLE MODIFICATION
The most successful but difficult therapy to “administer” is weight loss. Weight loss is most successful because it brings about the greatest global improvement in cardiovascular risks, insulin sensitivity, and menstrual patterns; it is most difficult because of the compliance issue. Weight loss should not be rapid or drastic but should be achieved through consistent lifestyle modification that includes gentle exercise, intake of dietary carbohydrates with a low glycemic index, and a reduced intake of fats and simple sugars. Severe dietary deprivation is uniformly unsuccessful.
No single food plan is recommended, but frequent feedings (four to six times per day) are important to avoid hypoglycemia and hunger. Hypoglycemia leads to cravings and poor food choices that, in turn, result in simple sugar intake and reactive hyperinsulinemia. In the experience of the author, most women respond well to the admonition to eat more food more frequently to lose weight.
In addition to the metabolic improvements, successful weight loss has the benefit of improving body image, reducing depression, and restoring a sense of control. Programs that have targeted this issue alone have achieved pregnancy rates as high as 60 percent without medical intervention.35 There are no data to support the use of lipid-lowering drugs in women younger than 40 years, but dietary regulation should be encouraged. Family physicians should monitor blood pressure elevations and smoking cessation for further cardiovascular protection.
Knowledge gained over the past 60 years has carried PCOS beyond the realm of gynecology and infertility, and warrants heightened attention from physicians who will focus on the functional abnormalities that have serious long-term consequences. It is important to recognize that PCOS is an entity with a long lifespan, requiring “control” rather than “cure,” and that therapies will change with the stage of life (Figure 1). A considerable armamentarium of therapies exists for the family physician, using specialty referral for patients with intractable vaginal bleeding, infertility, or dermatologic problems.