A more recent article on diabetic peripheral neuropathy is available.
Am Fam Physician. 2005;71(11):2123-2128
Patient information: See related handout on diabetic neuropathy, written by the authors of this article.
Diabetic neuropathy is a debilitating disorder that occurs in nearly 50 percent of patients with diabetes. It is a late finding in type 1 diabetes but can be an early finding in type 2 diabetes. The primary types of diabetic neuropathy are sensorimotor and autonomic. Patients may present with only one type of diabetic neuropathy or may develop combinations of neuropathies (e.g., distal symmetric polyneuropathy and autonomic neuropathy). Distal symmetric polyneuropathy is the most common form of diabetic neuropathy. Diabetic neuropathy also can cause motor deficits, silent cardiac ischemia, orthostatic hypotension, vasomotor instability, hyperhidrosis, gastroparesis, bladder dysfunction, and sexual dysfunction. Strict glycemic control and good daily foot care are key to preventing complications of diabetic neuropathy.
Diabetic neuropathy can affect any part of the nervous system. This nerve disorder should be suspected in all patients with type 2 diabetes and in patients who have had type 1 diabetes for more than five years.1–4 In some instances, patients with diabetic neuropathy have few complaints, but their physical examination reveals mild to moderately severe sensory loss.2,5 Idiopathic neuropathy has been found to precede the onset of type 2 diabetes or to occur as an early finding in the disease.2–5
| Key clinical recommendation | Label | References |
|---|---|---|
| Tight glycemic control can prevent, delay, or slow the progression of diabetic neuropathy in patients with type 1 diabetes. | B | 17,20 |
| Patients with diabetes should be educated about proper foot care and should check their feet daily. | C | 22,23 |
| All patients with diabetes should have an annual foot examination by a health care professional. | C | 24 |
Classification of Diabetic Neuropathy
The primary types of diabetic neuropathy are sensorimotor and autonomic (Table 1). A patient may have only one type of neuropathy or might develop different combinations of neuropathies.
| Sensorimotor neuropathy | |
| Distal symmetric polyneuropathy | |
| Focal neuropathy | |
| Diabetic mononeuropathy (cranial, truncal, peripheral nerves) | |
| Mononeuropathy multiplex | |
| Diabetic amyotrophy | |
| Autonomic neuropathy | |
| Hypoglycemic unawareness | |
| Abnormal pupillary function | |
| Cardiovascular autonomic neuropathy | |
| Vasomotor neuropathy | |
| Sudomotor neuropathy (sweat glands) | |
| Gastrointestinal autonomic neuropathy | |
| Gastric atony | |
| Diabetic diarrhea or constipation | |
| Fecal incontinence | |
| Genitourinary autonomic neuropathy | |
| Bladder dysfunction | |
| Sexual dysfunction | |
Sensory neuropathies can be classified as distal symmetric polyneuropathy, focal neuropathy (e.g., diabetic mononeuropathy), and diabetic amyotrophy. Motor neuropathies are identified by the muscles that are involved. Autonomic neuropathies may be classified by the system that is affected (e.g., endocrine, gastrointestinal, genitourinary). Symptoms of various forms of diabetic neuropathy are listed in Table 2.
| Sensorimotor neuropathy |
| Muscular symptoms: muscle weakness (not fatigue), atrophy, balance problems, ataxic gait |
| Sensory symptoms: pain, paresthesia, numbness, paralysis, cramping, nighttime falls, antalgic gait |
| Autonomic neuropathy |
| Cardiovascular symptoms: exercise intolerance, fatigue, sustained heart rate, syncope, dizziness, lightheadedness, balance problems |
| Gastrointestinal symptoms: dysphagia, bloating, nausea and vomiting, diarrhea, constipation, loss of bowel control |
| Genitourinary symptoms: loss of bladder control, urinary tract infection, urinary frequency or dribbling, erectile dysfunction, loss of libido, dyspareunia, vaginal dryness, anorgasmia |
| Sudomotor (sweat glands) symptoms: pruritus, dry skin, limb hair loss, calluses, reddened areas |
| Endocrine symptoms: hypoglycemic unawareness |
| Other symptoms: difficulty driving at night, depression, anxiety, sleep disorders, cognitive changes |
Once a careful history and a thorough physical examination have established the presence of diabetic neuropathy (Table 3), assessment strategies can help in management.
| History | |
| Screen for symptoms of diabetic neuropathy (see Table 2). | |
| Review diabetes history, disease management, daily glycemic records, and previous hemoglobin A1C levels. | |
| Identify any family history of diabetes or neuropathy. | |
| Review medication history (including use of over-the-counter products and herbal or homeopathic products) and environmental exposures. | |
| Review for other causes of neuropathy, including vitamin B12 deficiency, alcoholism, toxic exposures, medications, cancers, and autoimmune disease. | |
| Physical examination | |
| Vital signs and pain index | |
| Supine and standing blood pressure for postural hypotension | |
| Cardiovascular examination to look for arrhythmias, absent or diminished pulses, edema, or delayed capillary refilling | |
| Cutaneous examination to look for extremity hair loss, skin or nail changes (including callus), and pretrophic (red) areas, especially between toes | |
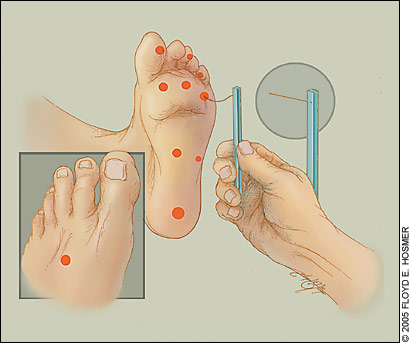
| Neurologic examination using the 5.07 Semmes-Weinstein (10-g) nylon filament test (10-g monofilament test) | |
| Inspection of feet for asymmetry, loss of arch height, or hammer toes | |
| Evaluation of all positive screening findings | |
| Annual diabetes evaluation | |
| Evaluation for neuropathy as discussed above | |
| Sensorimotor examination and evaluation of cranial nerves, muscle strength, and range of motion | |
| Document distribution, intensity, and type of sensory or motor deficits. | |
| Evaluate small nerve fibers with temperature, light touch, or pinprick testing. | |
| Test large nerve fibers by vibratory sensation, position sense, muscle strength, sharp-dull discrimination, and two-point discrimination. | |
| Autonomic examination, including orthostatic blood pressure measurements | |
| Consider heart rate variability tests and electrocardiography if sensory neuropathy is present or symptoms warrant further evaluation. | |
| Consider heart rate variability tests in the patient who has had type 1 diabetes for 10 years or type 2 diabetes for five years; consider cardiac stress testing before the patient starts an exercise program. | |
Sensorimotor Neuropathy
In sensory nerve damage, the nerves with the longest axons usually are affected first, resulting in a stocking-and-glove distribution. Small fiber damage affects sensation of temperature, light touch, pinprick, and pain. Large fiber damage diminishes vibratory sensation, position sense, muscle strength, sharp-dull discrimination, and two-point discrimination. Polyradiculopathies and severe band-like abdominal pain also may occur.
Polyradiculopathy may be identified by electromyography or a sensory examination that shows altered sensation along the course of the nerve trunk. Bilateral thigh pain or weakness with atrophy of the iliopsoas, quadriceps, and adductor muscles also may be present. Physical findings involving the L2, L3, and L4 nerve roots or an abnormal electromyograph should alert the physician to the presence of polyradiculopathy.
When evaluating for sensorimotor neuropathy, it is important to ask the patient about recent falls and to look for loss of Achilles and patellar tendon reflexes, gait ataxia, and balance problems.
DISTAL SYMMETRIC POLYNEUROPATHY
Distal symmetric polyneuropathy, the most common form of diabetic neuropathy, affects approximately 40 percent of patients who have had diabetes for 25 years or longer. Most often, this neuropathy develops in the feet. The course is chronic and progressive; in rare cases, however, the neuropathy resolves spontaneously in six to 12 months.
Distal symmetric polyneuropathy predisposes patients to variable pain, motor dysfunction, nerve palsies, ulcers, burns, infections, gangrene, and Charcot’s disease. Affected patients also may develop neuropathic cachexia syndrome, which includes anorexia, depression, and weight loss. When testing is performed in patients with distal symmetric polyneuropathy and initial skin ulceration, almost 70 percent deny hypoesthesia, and about 50 percent can sense a cotton wisp and pinprick.6
FOCAL NEUROPATHY
Diabetic mononeuropathy has an acute onset and usually is asymmetric. Cranial, truncal, and peripheral nerves are involved. The neuropathy generally resolves spontaneously in three to 12 months, but in rare cases it may last for years.
Patients with diabetic mononeuropathy may develop visual changes or muscle weakness involving cranial nerves III, IV, and VI, as well as Bell’s palsy. Cranial nerve III involvement results in ophthalmoplegia, ptosis, and diplopia with sparing of pupillary function. The median, radial, and lateral popliteal nerves are the most common sites of peripheral nerve involvement.
Occasionally, nerve palsies affect several unilateral nerves. When multiple nerves are involved, the term “mononeuropathy multiplex” is used. Vasculitis should be ruled out as a cause of the symptoms.
DIABETIC AMYOTROPHY
Diabetic amyotrophy, also known as femoral neuropathy or proximal motor neuropathy, usually is bilateral and frequently is associated with weight loss. This condition causes thigh muscle weakness, as well as variable pain and loss of the patellar reflex. Diabetic amyotrophy tends to occur more often in older male patients with type 2 diabetes.
Thigh muscle atrophy is prominent, disabling, and usually limited to the iliopsoas, quadriceps, and adductor muscles. Less often, the anterolateral calf muscles are involved. Recovery usually is spontaneous in six to 12 months, but amyotrophy may recur. Increasing circumferential thigh measurements may not indicate recovery because muscle can be replaced by fatty tissue.
Diabetic Autonomic Neuropathy
Diabetic autonomic neuropathy can develop in patients with type 1 or type 2 diabetes. Although autonomic neuropathy may occur at any stage of diabetes,3,4 usually it develops in patients who have had the disease for 20 years or more with poor glycemic control. The reported prevalence of diabetic autonomic neuropathy varies widely, depending on the cohort studied and the methods of assessment.7
In autonomic disease, the sympathetic, parasympathetic, and enteric nerves are affected. Myelinated and unmyelinated nerve damage is found. Diabetic autonomic neuropathy may lead to hypoglycemic unawareness and increased pupillary latency. Many investigators have considered autonomic neuropathies to be irreversible. However, cardiac sympathetic dysinnervation has been shown to regress with tight glycemic control.8
CARDIOVASCULAR AUTONOMIC NEUROPATHY
The risk of cardiovascular events is at least two to four times higher in patients with diabetes.9 Cardiovascular neuropathy is a result of damage to vagal and sympathetic nerves. Clinical findings may include exercise intolerance, persistent sinus tachycardia, no variation in heart rate during activities, and bradycardia. Baroreceptor disease contributes to supine hypertension.
In a patient with type 1 diabetes, an autonomic imbalance may result in a prolonged QT interval on the electrocardiogram (ECG), which may predispose the patient to life-threatening cardiac arrhythmias and sudden death.7 Diabetic neuropathy also can reduce appreciation of ischemic pain, which may delay appropriate medical therapy and lead to death.7
Orthostatic blood pressure measurements may be used to evaluate cardiovascular autonomic dysfunction.10 Stress testing should be considered before any patient with diabetes starts an exercise program.
VASOMOTOR NEUROPATHY
Vasomotor neuropathy frequently causes orthostatic hypotension by affecting the splanchnic and peripheral vascular beds. Symptoms of syncope or dizziness often have day-to-day variability and may be exacerbated by insulin therapy or the postprandial state, in which there is splanchnic shunting of blood. The evaluation should include vital signs, an ECG, and orthostatic blood pressure measurements.
In diabetic neuropathy, neuronal input to the peripheral vasculature is decreased or absent. Resultant peripheral vasomotor instability can manifest as persistent excess peripheral circulation (hyperemia) and peripheral edema. Loss of sympathetic tone in the blood vessels results in maximal vasodilation, which can lead to arteriovenous shunting in the soft tissue and bone. Increased blood flow through the bone causes calcium to wash from the cortical stores. Defective bone homeostasis and bone demineralization may result.11
The occurrence of peripheral vasomotor instability and peripheral sudomotor neuropathy is termed “autosympathectomy.” The patient with autosympathectomy has peripheral vasomotor reflexes similar to those in a nondiabetic patient after sympathectomy. The mechanism by which the body senses and responds to changes in blood pressure by reflex vasodilation or contraction of peripheral vessels is impaired. Autosympathectomy and distal symmetric polyneuropathy are considered necessary for the development of Charcot’s disease (diabetic neuropathic arthropathy).12
SUDOMOTOR NEUROPATHY
Sudomotor neuropathy may cause hyperhidrosis and heat intolerance in the upper torso or anhidrosis in the lower extremities. Temperature elevation is rare, but sometimes occurs. The skin of the extremities may feel pruritic and may display thinning, hair loss, dryness, flaking, cracks, increased callus formation, and nail dystrophies. These skin changes increase the risk of ulceration.
GASTROINTESTINAL AUTONOMIC NEUROPATHY
Gastrointestinal autonomic neuropathy may cause paresis anywhere in the digestive tract, with damage to small myelinated and unmyelinated splanchnic nerves. Reduced contraction amplitudes of the tubular esophagus may cause mild dysphagia. Motility studies, such as scintigraphy after a radiolabeled meal, are helpful in the evaluation of nausea, vomiting, early satiety, and delayed gastric emptying.
Diabetic diarrhea is caused by increased or uncoordinated transit time in the small intestine, bacterial overgrowth, or increased intestinal secretion.13 Stool cultures and flexible sigmoidoscopy may be helpful in excluding other causes of diarrhea, such as parasitic infection, colon cancer or polyps, celiac sprue, and inflammatory bowel disease.
Decreased transit time in the large intestine may cause constipation or impacted stool. Abdominal radiography or computed tomography may reveal megacolon or fecal impaction. Neuropathic fecal incontinence also may occur in patients with gastrointestinal autonomic neuropathy. A reduced threshold of conscious rectal sensation is manifested by a decreased resting anal sphincter pressure.14
DIABETIC BLADDER DYSFUNCTION
In patients with diabetic bladder dysfunction, inability to sense a full bladder and detrusor muscle hypoactivity cause retention and incomplete voiding of urine. These conditions can progress to overflow incontinence and urinary tract infections. Hyperglycemia alone also can cause increased urine production and incontinence.
The evaluation of the patient with diabetes who has bladder dysfunction should begin with a review of medications. Drugs that impair detrusor contractility and increase urethral tone include calcium channel blockers, anticholinergics, alpha- and beta-adrenergic agonists, narcotics, antidepressants, and antipsychotics. Further work-up should include a patient’s voiding record, post-void residual testing, and urinalysis. Cystometric and urodynamic studies confirm the diagnosis.7
ERECTILE DYSFUNCTION
Erectile dysfunction can occur at an early age in men with diabetes.15 It develops in 35 percent of men with diabetes between 20 and 59 years of age and 65 percent of men with diabetes 60 years or older.16 The primary cause is pelvic plexus neuropathy; a decrease in nitric oxide, which is required to initiate an erection, contributes to the condition.
Routine screening is important because erectile dysfunction may occur before the development of other autonomic signs. The evaluation of erectile dysfunction includes a sexual history, a genital examination, a serum testosterone level, and prolactin and thyrotropin levels.
FEMALE SEXUAL DYSFUNCTION
In women, diabetic neuropathy may cause vaginal dryness, decreased perineal sensation, dyspareunia, reduced libido, or anorgasmy.7 Routine screening should be performed because sexual dysfunction may precede other autonomic signs. A detailed sexual history, pelvic examination, and urinalysis help rule out other diagnoses.
Preventing Complications of Diabetic Neuropathy
GLYCEMIC CONTROL
The Diabetes Control Complications Trial (DCCT)17,20 demonstrated that tight glycemic control may result in a 60 percent reduction in the risk of developing clinical neuropathy. The American Diabetes Association (ADA)19 has adopted the DCCT-established standards for tight glycemic control in patients with type 1 diabetes, 13 to 39 years of age at initiation of the study: a mean blood glucose level of 155 mg per dL (8.6 mmol per L) and a hemoglobin A1C value of 7.2 percent.17,19,20 In patients with type 2 diabetes, the A1C value should be less than 7.0 percent, and peak postprandial plasma glucose levels should be less than 180 mg per dL (10.0 mmol per L). No clinical trial data are available on the effects of glycemic control in older patients, in young children, or in patients with advanced complications.
The American Association of Clinical Endocrinologists21 recommends an A1C value of less than 6.5 percent in patients with type 1 or type 2 diabetes.
FOOT CARE
Daily foot care is essential for preventing complications of diabetic neuropathy (see patient information handout). Patients should be instructed to inspect their feet daily for dry or cracking skin, fissures, plantar callus formation, and signs of infection between the toes and around the toenails.22,23 Application of topical ointments to intertriginous areas should be avoided.11
Properly fitted footwear is crucial. New shoes are a common cause of ulceration and should be broken in slowly. Patients also should avoid sources of possible trauma, such as walking barefoot, cutting nails incorrectly, and exposing their feet to hot objects or chemicals such as hydrogen peroxide, iodine, or astringents (e.g., witch hazel).
At each visit, the physician should examine the patient’s feet visually to detect evidence of neuropathy or early lesions. The ADA24 recommends a thorough annual foot examination by a health care professional for all patients with diabetes. The feet should be checked for skin breaks, red or callused areas, decreased or absent pedal pulses, and delayed capillary refilling, bony deformities, and protective sensation. Protective sensation is assessed by the 5.07 Semmes-Weinstein (10-g) nylon filament test (10-g monofilament test; Figure 1).11,25