
Am Fam Physician. 2007;76(6):801-808
Patient information: See related handout on gout, written by the author of this article.
Author disclosure: Nothing to disclose.
Arthritis caused by gout (i.e., gouty arthritis) accounts for millions of outpatient visits annually, and the prevalence is increasing. Gout is caused by monosodium urate crystal deposition in tissues leading to arthritis, soft tissue masses (i.e., tophi), nephrolithiasis, and urate nephropathy. The biologic precursor to gout is elevated serum uric acid levels (i.e., hyperuricemia). Asymptomatic hyperuricemia is common and usually does not progress to clinical gout. Acute gout most often presents as attacks of pain, erythema, and swelling of one or a few joints in the lower extremities. The diagnosis is confirmed if monosodium urate crystals are present in synovial fluid. First-line therapy for acute gout is nonsteroidal anti-inflammatory drugs or corticosteroids, depending on comorbidities; colchicine is second-line therapy. After the first gout attack, modifiable risk factors (e.g., high-purine diet, alcohol use, obesity, diuretic therapy) should be addressed. Urate-lowering therapy for gout is initiated after multiple attacks or after the development of tophi or urate nephrolithiasis. Allopurinol is the most common therapy for chronic gout. Uricosuric agents are alternative therapies in patients with preserved renal function and no history of nephrolithiasis. During urate-lowering therapy, the dose should be titrated upward until the serum uric acid level is less than 6 mg per dL (355 μmol per L). When initiating urate-lowering therapy, concurrent prophylactic therapy with low-dose colchicine for three to six months may reduce flare-ups.
Gouty arthritis accounted for an estimated 3.9 million outpatient visits in the United States in 2002.1 Unlike other rheumatic diseases, the etiology of gout is well characterized; its pathophysiology is well understood; the disease is easily diagnosed; and effective, inexpensive therapies are available. However, data indicate that even with universal health care coverage, quality of treatment may be suboptimal in up to one half of patients with gout.2 The National Health and Nutrition Examination Survey III showed that the overall prevalence of self-reported, physician-diagnosed gout was 2 percent in men older than 30 and in women older than 50.3 No published population studies of gout have used identification of intra-articular crystals as the diagnostic criterion. Recent insurance claims data show that the prevalence increased annually by two cases per 1,000 persons between 1990 and 1999.4 Increasing rates of obesity5 and an aging population with chronic medical conditions such as diuretic-treated hypertension may contribute to increasing gout diagnoses.
| Clinical recommendation | Evidence rating | References |
|---|---|---|
| Serum uric acid measurements are useful in the evaluation of gout; however, they should not be used alone to confirm or exclude the diagnosis. | C | 12,17,19 |
| Nonsteroidal anti-inflammatory drugs, corticosteroids, and colchicine are effective treatments for acute gout. | B | 20,22–25 |
| In patients with gout, modifiable risk factors such as obesity, diuretic use, high-purine diet, and alcohol intake should be addressed. | B | 13,14,17 |
| Urate-lowering therapy is recommended for patients with recurrent gout attacks, tophi, or ongoing arthropathy with joint damage seen on a radiograph. | C | 20 |
| When initiating urate-lowering therapy, prophylaxis with low-dose colchicine for three to six months may reduce the risk of flare-ups. | B | 20,28 |
| During urate-lowering therapy, the target serum uric acid level is less than 6 mg per dL (355 μmol per L). | B | 20,29 |
| Allopurinol (Zyloprim) is the recommended first-line agent for urate-lowering therapy. | C | 20 |
Epidemiology and Pathophysiology
Uric acid is a metabolic by-product of purine catabolism. In most mammals, the urate oxidase (uricase) enzyme converts uric acid to allantoin, leading to very low serum uric acid levels (i.e., less than 1 mg per dL [60 μmol per L]).6 In humans and the great apes, however, the genes for uricase have mutated and become dysfunctional.6 Hyperuricemia (i.e., serum uric acid concentration greater than 6.5 mg per dL [385 μmol per L]) is common in the general population and is often caused by a combination of a high purine diet, alcohol use, diuretic therapy, and reduced renal clearance.
Gout is caused by altered purine metabolism leading to hyperuricemia. When the local solubility limits of uric acid are exceeded, monosodium urate crystal deposition in the joints, kidneys, and soft tissues causes clinical manifestations, including arthritis, soft tissue masses (i.e., tophi), nephrolithiasis, and urate nephropathy. Asymptomatic hyperuricemia is common and usually does not lead to clinical gout.
The relationship between hyperuricemia and cardiovascular disease is controversial. A small, nonblinded, randomized controlled trial found that patients who received allopurinol (Zyloprim) had improved postoperative outcomes following coronary artery bypass surgery.7 Several studies, including Framingham cohorts8 and a small, open-label, crossover trial,9 have found an association between hyperuricemia and hypertension. However, a recent meta-analysis of prospective studies found no association between hyperuricemia and adverse cardiovascular outcomes after adjustment for confounding variables, such as patient weight, blood pressure, cigarette use, and sex.10 The clinical diagnosis of gout has also been associated with adverse cardiovascular outcomes. A recent study found that, after adjusting for confounding variables, there was a small independent risk of acute myocardial infarction in men with gout.11
Risk Factors
Gouty arthritis is caused by intense inflammation secondary to monosodium urate crystal deposition in joints. Local factors that contribute to this deposition are changes in pH level (e.g., from perioperative ketosis in surgical patients); lower body temperature, explaining nocturnal attacks; and the level of articular dehydration (e.g., from initiation of diuretic therapy). However, most persons with elevated serum uric acid levels do not develop gout. Data show that the annual incidence of gout is 0.5 percent in persons with a uric acid level between 7 and 8.9 mg per dL (415 and 530 μmol per L), and the annual incidence is 4.5 percent in those with a level of 9 mg per dL (535 μmol per L) or greater.12
Any systemic factor that increases the risk of hyperuricemia can also increase the risk of symptomatic gout. Modifiable risk factors include a high-purine diet, alcohol use, obesity, and diuretic therapy. Data show an increased risk of gout with consumption of red meat and seafood but show a potentially protective effect with consumption of dairy products.13,14 Common triggers for acute gout are infection; intravenous contrast media; acidosis; and rapid fluctuations in serum uric acid concentrations such as with trauma, surgery, psoriasis flare-ups, initiation of chemotherapy, diuretic therapy, and stopping or starting allopurinol.
Clinical Presentation
ACUTE GOUT
Acute gouty arthritis most commonly begins with involvement of a single joint or multiple joints in the lower extremities, most commonly the first metatarsophalangeal (i.e., podagra), midtarsal, ankle, or knee joints. Pain, erythema, and swelling often begin in the early morning and increase and peak within 24 to 48 hours. The pain is severe, and patients often cannot wear socks or touch bedsheets during flare-ups. Even without treatment, the attacks typically subside within five to seven days.
Acute gout sometimes resembles cellulitis and can lead to skin desquamation over the inflamed area. Gout can also cause acute bursitis or tenosynovitis of periarticular structures. Acute polyarticular gout is less common but has a more dramatic presentation. Acute gout can cause a high fever and leukocytosis (sometimes more than 40,000 white blood cells per mm3 [40 × 109 per L]) and may be difficult to distinguish from acute septic arthritis. If the diagnosis is unclear, bacteriologic cultures of the synovial fluid and blood are warranted, and corticosteroid injections should be deferred.
CHRONIC GOUT
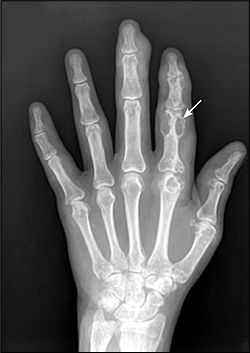
Frequent, recurrent acute attacks often cause chronic tophaceous gout. Tophi are deposits of monosodium urate crystals in soft tissue that may occur in the helix of the ear, over olecranon processes, and over interphalangeal joints. Tophi can occur over osteoarthritic Heberden's or Bouchard's nodes in the distal and proximal interphalangeal joints, especially in older women.15 Tophaceous gout may lead to significant morbidity and, if untreated, can cause joint erosion and destruction (Figure 1). Occasionally, polyarticular tophaceous gout presents as subcutaneous nodules that can mimic rheumatoid arthritis. In this case, the presence of monosodium urate crystals in the nodule aspirate can confirm gout.
Diagnosis
Classification criteria to aid in the diagnosis of gout have been proposed by the American College of Rheumatology (Table 1),16 and a consensus panel of experts from the European League Against Rheumatism (EULAR) has reviewed the evidence and made recommendations for diagnosing gout.17 The main differential diagnosis (Table 2) of acute gout is pseudogout (calcium pyrophosphate deposition disease) and septic arthritis.
| Gout may be diagnosed if one of the following criteria is present: | |
| Monosodium urate crystals in synovial fluid | |
| Tophi confirmed with crystal examination | |
| At least six of the following findings: | |
| Asymmetric swelling within a joint on a radiograph | |
| First metatarsophalangeal joint is tender or swollen (i.e., podagra) | |
| Hyperuricemia | |
| Maximal inflammation developed within one day | |
| Monoarthritis attack | |
| More than one acute arthritis attack | |
| Redness observed over joints | |
| Subcortical cysts without erosions on a radiograph | |
| Suspected tophi | |
| Synovial fluid culture negative for organisms during an acute attack | |
| Unilateral first metatarsophalangeal joint attack | |
| Unilateral tarsal joint attack | |
| Diagnosis | Joint distribution | Synovial fluid findings | |||
|---|---|---|---|---|---|
| WBC count* | Gram stain/culture | Synovial fluid crystals† | Radiography findings | ||
| Gout | Lower extremities: metatarsophalangeal, midtarsal, or knee joints; initial attacks may be less common in upper extremities | 2,000 to 50,000 per mm3 (2 × 109 to 50 × 109 per L) | Negative | Needle shaped, negative birefringence | Acute: asymmetric swelling |
| Chronic: periarticular erosions with overhanging edges | |||||
| Pseudogout (calcium pyrophosphate deposition disease) | Knee, wrist, or first metatarsophalangeal | 2,000 to 50,000 per mm3 | Negative | Rhomboid shaped, weak positive birefringence | Soft tissue swelling, chondrocalcinosis (calcification of cartilage) |
| Septic arthritis | Knee is most commonly involved (may be any joint distribution) | < 50,000 per mm3 | Positive | No crystals | Joint effusion; radiography results otherwise normal early in the disease |
Table 3 presents data for the accuracy of key elements in the diagnosis of gout.16–18 The presence of podagra or tophi strongly supports a gout diagnosis. The presence of monosodium urate crystals in synovial fluid is confirmatory, although a synovial fluid analysis is not always feasible. In the appropriate clinical scenario, a patient with hyperuricemia and classic podagra can be diagnosed and treated empirically. However, if a gout diagnosis is in question, synovial fluid analysis should be attempted. Serum uric acid measurements are not sufficient for confirming or ruling out gout because they may be normal during an acute attack.12,17,19
A 24-hour urine collection to detect uric acid excretion is not routinely performed. Collection and dietary restrictions are difficult, and most patients receive allopurinol for chronic urate-lowering therapy regardless of the cause of hyperuricemia.
Treatment
The goals of gout treatment are symptom control for acute attacks, risk factor modification, and pharmacotherapy to prevent recurrence and chronic sequelae. Recommendations from the EULAR guideline for the treatment of gout are summarized below.20
THERAPY FOR ACUTE ATTACKS
| Therapy/dosing | Cautions | Comments | |
|---|---|---|---|
| NSAIDs | Use with caution in older patients and in patients with renal insufficiency, heart failure, peptic ulcer disease, or liver disease and in those receiving anticoagulation therapy | Any NSAID is effective | |
| Indomethacin (Indocin), 50 mg three times daily for four to 10 days | |||
| Naproxen (Naprosyn), 500 mg twice daily for four to 10 days | |||
| Sulindac (Clinoril), 200 mg twice daily for four to 10 days | |||
| Corticosteroids | Avoid in patients with joint sepsis and use cautiously in patients with diabetes | Intra-articular therapy may be the treatment of choice if only one or two accessible joints are involved | |
| Prednisone, 20 to 40 mg daily for two or three days, then taper over 10 to 14 days | |||
| Intra-articular methylprednisolone (Depo-Medrol), one 20- to 40-mg dose | |||
| Intramuscular methylprednisolone, one 80- to 120-mg dose | |||
| Colchicine, 0.6 mg orally two or three times daily | Avoid in patients with severe renal or hepatic impairment because it can lead to bone marrow suppression and neuromyopathy | Avoid intravenous use; best if used within the first 24 hours of the attack; the most common adverse effects are nausea, vomiting, and diarrhea; reduce the dosage in older patients | |
| Suggested renal dosing (based on creatinine clearance): | |||
| > 50 mL per minute (0.83 mL per second): 0.6 mg twice daily | |||
| 35 to 50 mL per minute (0.58 to 0.83 mL per second): 0.6 mg daily | |||
| 10 to 34 mL per minute (0.17 to 0.57 mL per second): 0.6 mg every two or three days | |||
| < 10 mL per minute (0.17 mL per second): avoid | |||
Nonsteroidal anti-inflammatory drugs22,23 or corticosteroids24 are first-line therapies for acute gout, depending on patient comorbidities. Although colchicine is an effective second-line therapy, in higher doses the risks of adverse effects outweigh the benefits.25 Occasionally, these therapies may need to be supplemented by short-acting opioids such as hydrocodone (Hycodan) and oxycodone (Roxicodone). All medications should be used cautiously in older persons, in whom the threshold of toxicity is lower.
URATE-LOWERING THERAPY FOR CHRONIC GOUT
About 60 percent of persons who experience a gout attack will have another attack within 12 months.26 Therefore, nonpharmacologic treatment of hyperuricemia should begin with the first gout attack and should initially focus on modifiable risk factors such as diet (i.e., less red meat and seafood, more dairy) and alcohol intake. Substitution of diuretic therapy with other antihypertensives reduces hyperuricemia in many patients.13,14,17
Urate-lowering pharmacotherapy (Table 521,27) using a xanthine oxidase inhibitor or uricosuric agent is recommended for patients with more than two gouty attacks per year, in patients with tophi, and in patients with joint damage seen on a radiograph.20 However, this therapy should not commence until the acute phase of gout has completely resolved because fluctuations in serum uric acid levels will exacerbate the inflammatory process. When initiating urate-lowering therapy, concurrent prophylaxis with low-dose colchicine (0.6 to 1.2 mg daily) for three to six months has been shown to reduce the risk of flare-ups.28 The target serum uric acid level is less than 6 mg per dL (355 μmol per L),29 and doses of the urate-lowering therapy should be titrated upward until this target is reached.
| Therapy/dosing | Cautions | Comments | Monthly cost (generic)* | ||
|---|---|---|---|---|---|
| Allopurinol (Zyloprim), 50 to 300 mg daily (maximal daily dosage: 800 mg) | May precipitate acute gout, hypersensitivity syndrome, or mild rash; avoid using with azathioprine (Imuran); interacts with warfarin (Coumadin) | Do not initiate until four to six weeks after an acute attack; concurrent prophylaxis with colchicine (0.6 mg once or twice daily for six months) may prevent flare-ups; titrate dose until the uric acid level is less than 6 mg per dL (355 μmol per L); continue therapy during acute flare-ups | Thirty 300-mg tablets: $34 (6 to 18) | ||
| Suggested initial daily renal dosing (based on creatinine clearance): | |||||
| ≥ 90 mL per minute (1.50 mL per second): 300 mg | |||||
| 60 to 89 mL per minute (1.00 to 1.49 mL per second): 200 mg | |||||
| 30 to 59 mL per minute (0.50 to 0.98 mL per second): 100 mg | |||||
| 10 to 29 mL per minute (0.16 to 0.48 mL per second): 50 to 100 mg | |||||
| < 10 mL per minute (0.16 mL per second): use very cautiously | |||||
| Probenecid, initially 250 mg twice daily, gradually titrated to 500 mg to 2 g per day | May precipitate acute gout, nephrolithiasis, gastrointestinal upset, or rash; modifies renal handling of other drugs; use cautiously with heparin | Maintain hydration (about 2 L per day); avoid using with low-dose aspirin; ineffective if creatinine clearance is less than 50 mL per minute | Sixty 500-mg tablets†: (59 to 131) | ||
| Febuxostat, 80 mg daily | Avoid in patients with hepatic impairment | Investigational medication not yet approved by the U.S. Food and Drug Administration | — | ||
Allopurinol is the first-line urate-lowering therapy. In patients with normal renal function, the initial dosage may be 300 mg daily, although many physicians advocate starting with a lower dosage (e.g., 50 to 100 mg) and then titrating upward by 50 to 100 mg every two to four weeks (maximal daily dosage: 800 mg) until the target serum uric acid level is reached.
In patients with renal insufficiency, the allopurinol dosage should be adjusted based on the estimated creatinine clearance. Approximately 2 to 5 percent of patients taking allopurinol have minor rashes and other adverse effects. Rarely, a severe hypersensitivity syndrome occurs with fever, toxic epidermal necrolysis, hepatitis, and eosinophilia; this syndrome has been shown to have a 20 percent mortality rate.27 Those intolerant of allopurinol may undergo desensitization30 or may take oxypurinol (the active metabolite of allopurinol), if available.
Uricosuric agents are second-line therapy for patients who are intolerant of allopurinol, or they may be used in combination with allopurinol in patients with refractory hyperuricemia. Probenecid is the uricosuric agent most often used in the United States. Uricosuric therapy is contraindicated in patients with a history of nephrolithiasis and is ineffective in those with a creatinine clearance of less than 50 mL per minute (0.83 mL per second). Losartan (Cozaar) and fenofibrate (Tricor) have uricosuric properties and may be useful adjunctive therapies for patients with gout, hypertension, and hyperlipidemia.31
NEWER THERAPEUTIC OPTIONS
Febuxostat (investigational drug not yet approved by the U.S. Food and Drug Administration) is a novel nonpurine, xanthine oxidase antagonist that was recently shown to be comparable with allopurinol in lowering uric acid levels.32 Compared with patients taking 300 mg of allopurinol daily, more patients taking 80 mg of febuxostat reached target uric acid levels. However, the allopurinol dosage could not be titrated, and the febuxo-stat group had a high dropout rate because of adverse effects. At 52 weeks, the groups had similar rates of gout flare-ups. Febuxostat is cleared primarily through the liver and may be useful in those with chronic renal insufficiency who have elevated uric acid levels despite renal dosing of allopurinol.
There has been growing interest in reducing total body urate load using a recombinant uricase enzyme (rasburicase [Elitek]) in patients with advanced tophaceous gout. This therapy has been available for the treatment of tumor lysis syndrome and has been used for refractory tophaceous gout.33 Long-term use is limited because of induction of antigenic responses. A pegylated uricase enzyme has been developed and is currently undergoing trials.34