
A more recent article on enuresis in children is available.
Am Fam Physician. 2008;78(4):489-496
Patient information: See related handout on enuresis, written by the author of this article.
Author disclosure: Nothing to disclose.
Enuresis is defined as repeated, spontaneous voiding of urine during sleep in a child five years or older. It affects 5 to 7 million children in the United States. Primary nocturnal enuresis is caused by a disparity between bladder capacity and nocturnal urine production and failure of the child to awaken in response to a full bladder. Less commonly, enuresis is secondary to a medical, psychological, or behavioral problem. A diagnosis usually can be made with a history focusing on enuresis and a physical examination followed by urinalysis. Imaging and urodynamic studies generally are not needed unless specifically indicated (e.g., to exclude suspected neurologic or urologic disease). Primary nocturnal enuresis almost always resolves spontaneously over time. Treatment should be delayed until the child is able and willing to adhere to the treatment program; medications are rarely indicated in children younger than seven years. If the condition is not distressing to the child, treatment is not needed. However, parents should be reassured about their child's physical and emotional health and counseled about eliminating guilt, shame, and punishment. Enuresis alarms are effective in children with primary nocturnal enuresis and should be considered for older, motivated children from cooperative families when behavioral measures are unsuccessful. Desmopressin is most effective in children with nocturnal polyuria and normal bladder capacity. Patients respond to desmopressin more quickly than to alarm systems. Combined treatment is effective for resistant cases.
Enuresis is defined as repeated, spontaneous voiding of urine during sleep in a child five years or older.1 Enuresis may be classified as primary or secondary, and monosymptomatic (uncomplicated) or nonmonosymptomatic (i.e., concomitant lower urinary tract symptoms are present). Table 1 summarizes the types of enuresis.1,2 Children with primary nocturnal enuresis are monosymptomatic, have no lower urinary tract symptoms other than nocturia, and have no history of bladder dysfunction.1
| Clinical recommendation | Evidence rating | References |
|---|---|---|
| Treatment for primary nocturnal enuresis begins with educating the child and parents about the condition; daytime symptoms should be actively identified and managed before addressing primary nocturnal enuresis; and, if identified, secondary causes should be treated appropriately. | C | 2, 17 |
| If primary nocturnal enuresis is not distressing to the child, treatment is unnecessary, although parents should be reassured about their child's physical and emotional health and counseled about eliminating guilt, shame, and punishment. | C | 2, 19 |
| An enuresis alarm is effective in children with monosymptomatic nocturnal enuresis; overlearning (i.e., encouraging the child to drink extra fluids before bedtime to improve bladder capacity) should be added when continence has been achieved for 14 consecutive nights. | A | 2, 8, 17, 19, 24 |
| Dry-bed training and bladder training alone are not recommended to treat primary nocturnal enuresis. | B | 17, 23 |
| Anticholinergics are useful in children with urgency, restricted bladder capacity from detrusor hyperactivity at night, and combined daytime wetting and nocturnal incontinence and in children who do not respond to desmopressin (DDAVP). | B | 8, 17, 28 |
| Desmopressin is most effective in children who have monosymptomatic enuresis with nocturnal polyuria and normal bladder capacity. | A | 2, 8, 30, 31 |
| Type | Characteristics |
|---|---|
| Primary enuresis (80 percent of cases) | Enuresis in a child who has never established urinary continence for more than six months |
| Secondary enuresis (20 percent of cases) | Resumption of enuresis after at least six months of urinary continence |
| Nocturnal enuresis | Enuresis that occurs during sleep |
| Daytime wetting | Urinary incontinence that occurs while the child is awake |
| Monosymptomatic (uncomplicated) enuresis | Enuresis without lower urinary tract symptoms other than nocturia and no history of bladder dysfunction |
| Nonmonosymptomatic enuresis | Enuresis with lower urinary tract symptoms (e.g., increase or decrease in voiding frequency, daytime wetting, urgency, hesitancy, straining, weak or intermittent stream, posturination dribbling, holding maneuvers,* sensation of incomplete emptying, lower abdominal or genital discomfort) |
Pathophysiology
Primary nocturnal enuresis is caused by a disparity between bladder capacity and nocturnal urine production and the child's failure to awaken in response to a full bladder.8 Factors associated with enuresis (Table 2) include nocturnal polyuria, detrusor instability, and an abnormally deep sleep pattern.2,8–12 A small subgroup of children with primary nocturnal enuresis have little or no arousal to bladder distention and exhibit uninhibited bladder contractions before voiding (i.e., detrusor-dependent enuresis).9
| Factor | Description |
|---|---|
| Bladder function8 | Decreased functional bladder capacity; inability to hold urine at night |
| Detrusor instability8,9 | Lack of arousal to bladder distention and uninhibited bladder contractions (occurs in a small subgroup of children) |
| Genetic factors10,11 | Increased incidence of enuresis in children if one or both parents have a history of enuresis; in the case of twins, both children are usually affected |
| Maturation delay2,8 | Delay in central nervous system maturation and in the development of language and motor skills |
| Nocturnal polyuria8 | Decreased nocturnal secretion of antidiuretic hormone causes nocturnal polyuria; blunted response to antidiuretic hormone |
| Psychological factors2,8,12 | Not common with primary nocturnal enuresis; more common with secondary enuresis; considered a regressive symptom in response to stress or trauma (e.g., parental divorce, sexual abuse, trauma at school, hospitalization, neglect) |
| Sleep disorders9 | Enuresis occurs in all stages of sleep; an abnormally deep sleep pattern may occur in children with enuresis |
A variety of medical and psychological disorders are associated with secondary enuresis (Table 32,6 ). Underlying psychological stressors are suspected when a child who has not had enuresis develops the condition during a period of stress.2 In one study, 11 percent of girls who had been sexually abused presented with enuresis.12 However, there is little, if any, association between sexual abuse and primary nocturnal enuresis.
Genetic influences on nocturnal enuresis are heterogenous and complex.10 A history of enuresis in parents increases the risk. When one or both parents have a history of enuresis, the incidence in children is 44 and 77 percent, respectively, compared with a 15 percent incidence in children whose parents do not have a history of enuresis.2 If one twin has enuresis, the other twin usually is also affected.10,11 An autosomal dominant mode of transmission with high penetrance is present in some families, and many possible locations for the responsible genes (in chromosomes 8, 12, 13, and 22) have been identified.10
Clinical Features
A cross-sectional study comparing patients presenting with primary and secondary nocturnal enuresis showed that frequency, urgency, nocturia, constipation, and daytime wetting are common in both forms of enuresis.13 Constipation, which occurs in more than 50 percent of children with secondary nocturnal enuresis and in nearly 75 percent of children with primary nocturnal enuresis, is also associated with daytime wetting.13 A more recent prospective cross-sectional study of children with primary nocturnal enuresis showed that more than one third of patients have constipation.14
Behavioral problems are uncommon in children with primary nocturnal enuresis, especially those older than 10 years. These problems are more common in children with daytime wetting and over seven times more common in children with secondary enuresis.15 Behavioral problems include depression, anxiety, social phobias, conduct disorders, and attention-deficit/hyperactivity disorder (ADHD). The association with ADHD is more pronounced in older children (nine to 12 years of age); children with ADHD are three times more likely to have persistent voiding problems.16 Children with enuresis are more likely than other children to have subclinical psychological symptoms (e.g., inferiority complex, shame, irritability, timidity, impatience, isolation). Parental stress (e.g., emotional, social, and financial stress; intolerance; frustration) may also occur.8
Evaluation
Most children with primary nocturnal enuresis require only an enuresis-focused history, physical examination, and urinalysis before initiation of treatment; imaging and urodynamic studies are rarely needed (Table 41,2,6,8,10,11,17 ). The history should include the onset, duration, and severity of enuresis; presence of daytime wetting, constipation, genitourinary symptoms, and neurologic symptoms; family history of enuresis; patient medical and psychosocial history; and details of previous treatment. The parents and child should be interviewed.2 A two-week baseline record of the enuresis pattern (bladder diary) is helpful in the assessment of enuresis severity and subsequent treatment response.2
| Diagnostic approach | Components |
|---|---|
| History (enuresis-specific) | Age at onset of enuresis, duration and severity of enuresis, duration of continence (enuresis is not diagnosed in children younger than five years; recurrence after at least six months of urinary continence suggests secondary enuresis)1,2 |
| Presence of lower urinary tract symptoms*(symptoms other than nocturia suggest nonmonosymptomatic and secondary enuresis)1 | |
| History of medical illness (e.g., diabetes mellitus, sleep apnea) may suggest nonmonosymptomatic enuresis6 | |
| Psychosocial history (psychological disturbances are present in one third of patients with secondary enuresis)8 | |
| Family history of enuresis (the condition is more common in patients with a family history; in the case of twins, both children are usually affected)2,10,11 | |
| Fluid-intake diary, bladder and stooling diary, frequency/volume chart (records help assess constipation, enuresis severity, and treatment response)2,8 | |
| Investigation and treatment history | |
| Red flags: dysuria, genital or rectal pain or discharge, straining to urinate, combined diurnal and nocturnal frequency with enuresis (suggests nonmonosymptomatic enuresis) | |
| Physical examination2,6,8,17 | Ears, nose, and throat examination to detect adenotonsillar hypertrophy |
| Abdominal examination to detect enlarged bladder or kidneys and fecal masses indicating encopresis | |
| Genital examination to detect hypospadias or epispadias, meatal stenosis, ectopic ureter, and labial adhesions | |
| Rectal examination to evaluate perianal and perineal sensation and rectal sphincter tone and to detect perianal excoriation and vulvovaginitis | |
| Focused neurologic evaluation, including gait, muscle tone, strength, and perineal sensation | |
| Red flags (indicate need for further investigation): adenotonsillar hypertrophy, spinal pathology (deformity, sacral dimple or hair tuft suggesting underlying spinal dysraphism), motor sensory loss and abnormal tendon reflexes in the lower limbs, enlarged bladder or kidneys, abnormal gait, signs of sexual abuse | |
| Urinalysis, urine culture†6 | Detection of urinary tract infection, diabetes mellitus, diabetes insipidus |
| Blood count, serum chemistry†6 | Blood urea nitrogen and serum creatinine levels to detect chronic renal failure, serum glucose levels to detect diabetes, hemoglobin electrophoresis to detect sickle cell disease, serum thyroid-stimulating hormone level to detect hyperthyroidism |
| Imaging studies†2,6,8 | Renal and bladder ultrasonography and voiding cystourethrography for a suspected structural abnormality, significant daytime wetting, or recurrent urinary infections to detect vesicoureteral reflux |
| Magnetic resonance imaging of the lumbosacral spine for suspected spinal dysraphism or abnormal neurologic examination findings | |
| Urodynamic studies†2,8 | Measurement of residual urine and cystometry to evaluate bladder dysfunction (dysfunctional voiding) |
The physical examination should include evaluation of the ears, nose, throat, abdomen, spine, genitalia, and rectum and a focused neurologic examination. In children with secondary or persistent enuresis, the possibility of sexual abuse must be considered. Signs suggestive of sexual abuse include bruising in areas that are typically protected (e.g., buttocks, back, trunk, inner thighs, cheeks, neck); multiple bruises; and patterned bruises (e.g., handprints, belt buckle, bite marks).18
Urinalysis and urine culture help detect infection. Select laboratory tests are useful in diagnosing causes of secondary enuresis (e.g., elevated serum glucose level from diabetes, elevated blood urea nitrogen and creatinine levels from chronic renal failure, low serum thyroid-stimulating hormone level from hyperthyroidism). Imaging and urodynamic studies are reserved for children with significant daytime symptoms, history or diagnosis of urinary tract infections, features suggesting structural renal abnormalities, or refractory cases.2,8
Treatment of Primary Nocturnal Enuresis
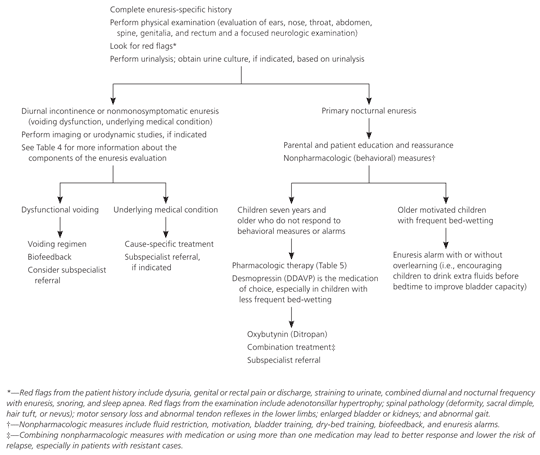
Treatment of primary nocturnal enuresis (Figure 1 and Table 52,8,17,19–35 ) should begin with educating the child and parents about the condition. The family should be reassured that primary nocturnal enuresis usually resolves spontaneously (15 percent annual cure rate).17 Secondary causes that were identified with the history, examination, or laboratory testing should be treated. Simple behavioral interventions are first-line treatment approaches. Arousal alarm systems and pharmacotherapy should be considered in older children who have greater social pressures and low self-esteem.
| Treatment | Indication and use | Effectiveness | Adverse effects |
|---|---|---|---|
| Nonpharmacologic* | |||
| Motivational therapy17,19–22 | Younger children with primary nocturnal enuresis Provides reassurance and emotional support, eliminates guilt, rewards continence(e.g., star or sticker charts) | Partial response in 75 percent of children; relapse in 5 percent of children | Frustration; reward system may worsen self-esteem if the child fails to have dry nights |
| Bladder training17,22 | Younger children with primary nocturnal enuresis Postpones voiding for longer periods | Minimal improvement | None |
| Dry-bed training17,23 | Younger children with primary nocturnal enuresis Includes waking children to void at specified intervals | Minimal improvement | Sleep deprivation |
| Enuresis alarm2,17,19,24 | Older, motivated children with primary nocturnal enuresis Awakens child in response to an alarm triggered by wetness | Effective in two thirds of children; dryness persists in 50 percent of children who continue to use the alarm | Sleep deprivation |
| Biofeedback25 | Older, motivated children with primary nocturnal enuresis and dysfunctional voiding Training for pelvic floor muscle relaxation | Primary nocturnal enuresis: 81 percent effective Lower urinary tract symptoms: 77 to 81 percent effective Constipation: 73 percent effective Daytime wetting: 58 percent effective | None |
| Pharmacologic† | |||
| Oral imipramine (Tofranil), 25 to 75 mg daily2,19,20,26,27 | Children with primary nocturnal enuresis | 40 to 60 percent effective; response occurs within days; most patients relapse after discontinuing treatment | Drowsiness, gastrointestinal upset, seizures, arrhythmia, overdose, lethargy, agitation, depression, sleep disturbance |
| Oral desipramine (Norpramin), 1 to 3 mg per kg daily | |||
| Oral oxybutynin (Ditropan), 2.5 to 5 mg three times daily8,17,28,29 | Children with urge incontinence or primary nocturnal enuresis and diurnal incontinence | 47 to 71 percent effective; better response if combined with desmopressin (DDAVP) | Dry mouth, blurred vision, constipation, dizziness, tachycardia, headache, nausea, gastrointestinal upset |
| Oral desmopressin, 0.2 to0.6 mg daily | Children with primary nocturnal enuresis | 60 to 70 percent effective; response occurs within days; 80 percent of patients relapse after discontinuing treatment | Headache, nasal congestion, epistaxis, sore throat, abdominal cramps, water intoxication, allergic reaction, hyponatremia, anorexia, nausea, visual disturbance, bad taste in the mouth |
| Intranasal desmopressin, 10 to 40 mcg daily2,8,30–35 | |||
Medication should be initiated in children seven years and older only if nonpharmacologic measures fail. Children who do not respond to one or more measures may benefit from combined treatment strategies (e.g., combining nonpharmacologic and pharmacologic treatment or multiple pharmacologic therapies). Children with persistent enuresis should be referred to a subspecialist. Presence of daytime wetting or abnormal voiding, straining or poor stream, genital abnormalities, or a history of urinary tract infections also indicates the need for referral.2
NONPHARMACOLOGIC OPTIONS
If primary nocturnal enuresis is not distressing to the child, treatment is not needed. However, parents should be reassured about their child's physical and emotional health and counseled about eliminating guilt, shame, and punishment.2,19 Treatment of primary nocturnal enuresis should be delayed until the child is able and willing to adhere to the treatment program and is rarely indicated in children younger than seven years. It may take months for a treatment program to be successful; therefore, the child must be highly motivated. Daytime symptoms should be actively identified and managed before addressing primary nocturnal enuresis.17 Treatment is considered successful when the child achieves continence for 14 consecutive nights within a 16-week period. Nonresponse to treatment is defined as less than a 50 percent decrease in enuresis; a 50 to 90 percent decrease suggests a partial response.1
Motivational therapy includes reassurance, emotional support, eliminating guilt, and encouraging the child to take responsibility for the enuresis (i.e., although the child did not cause the condition, he or she has a role in treating it).20 Simple behavioral interventions include awakening the child to void at times usually associated with bed-wetting; positive reinforcement for desired behavior (e.g., star or sticker charts for rewarding periods of continence); bladder training; and minimizing fluid and caffeine intake before bedtime. These methods are associated with significantly fewer wet nights, higher cure rates, and lower relapse rates compared with control groups. However, behavioral interventions have higher nonadherence rates and require significant parental involvement.21,22 Taking the child to the bathroom during the night is labor intensive and can frustrate parents. If reward systems are used, failure to achieve dry nights may worsen the child's self-esteem.19
Enuresis alarms (bells or buzzers) triggered by a moisture sensor in the bed pad or pajamas have long-term effectiveness2,19,24 Alarms condition children to awaken or contract their pelvic muscles. Most children require six to 16 weeks of treatment. Enuresis resolves in nearly two thirds of children during alarm use, and nearly one half of children who continue its use remain dry.24 No alarm system is superior to another.21 Sleep disruption is a problem with alarm use. Factors that predict a good response to enuresis alarms include a cooperative family, no coexisting emotional and behavioral problems, small bladder capacity, and frequent bed-wetting (four or more wet nights per week).8 Enuresis alarms should be considered in older, motivated children from cooperative families when behavioral measures are unsuccessful.17,19
Combining enuresis alarms with other behavioral modalities enhances treatment success. Adding over-learning (i.e., encouraging children to drink extra fluids before bedtime to improve bladder capacity) when continence has been achieved for 14 consecutive nights reduces relapse rates.24 Adding dry-bed training (i.e., awakening children at specified intervals until they learn to awaken on their own when necessary 17) is effective in 75 percent of children and reduces relapse rates compared with the use of an enuresis alarm alone.36 Combining an enuresis alarm with arousal training (i.e., rewarding children for awakening in response to the alarm) is effective in more than 90 percent of children.8
An alarm clock is a simple, inexpensive, safe, and modestly effective alternative to an enuresis alarm and does not require bed-wetting to evoke a conditioned response. Using the alarm clock to awaken the child for voiding when the bladder is full, but before incontinence occurs, or two to three hours after the child goes to sleep is effective in 62 to 77 percent of children. However, relapse rates three months after completion of treatment are the same as with an enuresis alarm.37
Full-spectrum home training includes behavioral interventions such as encouraging the child to remove soiled sheets and remake the bed, overlearning, dry-bed training, and bladder training. A systematic review showed that these measures led to minimal improvement when used alone; however, when used with an enuresis alarm, they reduced relapse rates.23
PHARMACOLOGIC OPTIONS
Pharmacologic therapies are not curative, but they decrease the frequency of enuresis or temporarily resolve symptoms over time until spontaneous resolution occurs. Options include anticholinergic agents (oxybutynin [Ditropan], hyoscyamine [Levsin]); tricyclic antidepressants (imipramine [Tofranil], desipramine [Norpramin]); and desmopressin (DDAVP). Of these therapies, only imipramine and oral desmopressin have been approved by the U.S. Food and Drug Administration for the treatment of enuresis in children.
Tricyclic antidepressants reduce bed-wetting by one wet night per week during treatment. Imipramine doses range from 25 mg for children older than six years (weighing 20 to 25 kg [44 lb, 1 oz to 55 lb, 2 oz]) to 50 to 75 mg for children older than 11 years. Some recommendations advise limiting the treatment period to three months (including gradual withdrawal).19 Imipramine, 25 mg, should be taken orally one hour before bedtime. If the response is not satisfactory after one or two weeks, the dose is increased to 50 mg in children seven to 12 years of age and up to 75 mg in older children.20 Most children relapse after discontinuing imipramine treatment.26
Parents should be warned about the potentially serious, dose-related adverse effects of tricyclic antidepressant use.26 Drowsiness, lethargy, agitation, depression, sleep disturbance, and gastrointestinal upset may occur. Rare adverse effects include seizures, cardiac arrhythmias, and death from accidental overdose.26,27 Pretreatment electrocardiography to identify underlying rhythm disorders is recommended.2
Anticholinergics (e.g., oxybutynin, 2.5 to 5 mg three times daily) decrease detrusor tone, frequency, and urgency and improve bladder capacity. Anticholinergic therapy may be used in children with primary nocturnal enuresis and daytime wetting (restricted bladder capacity caused by hyperactive detrusor muscle) and in patients who do not respond to desmopressin.8,17,28 Adverse effects include dry mouth, blurred vision, headache, nausea, dizziness, gastrointestinal upset, and tachycardia.29
Desmopressin, an analogue of vasopressin, reduces urine volume by reabsorbing water from the distal convoluted and collecting tubules. Sixty to 70 percent of children respond to treatment, although 80 percent relapse after discontinuing therapy.2,8,30 Desmopressin is recommended if the family is unwilling or unable to adhere to nonpharmacologic measures. The drug is most effective in children eight years and older who have monosymptomatic enuresis with nocturnal polyuria, normal bladder capacity, and less frequent bed-wetting.31
Desmopressin is available as an oral tablet (0.2 mg) or as a nasal spray (10 mcg per spray); its duration of action is 12 hours.2 Initial treatment is usually one tablet or one spray every night; the dosage is increased weekly to 0.6 mg or 40 mcg daily. Desmopressin therapy is combined with fluid restriction (less than 240 mL on nights when the drug is administered) and voiding before bedtime. Equivalent oral and intranasal doses have similar potency.30 Another option is rapid titration upward until continence is achieved within one to three days.32 The lowest effective dose of desmopressin should be used. Maintenance therapy of at least four to six weeks and a slow stepwise dose reduction over six to seven months decrease relapse rates after discontinuation of therapy.32
Adverse effects from desmopressin use occur in 5 percent of patients. Intranasal use may cause nasal congestion, epistaxis, sore throat, cough, or headaches. Systemic adverse effects from intranasal or oral use are rare and include allergic reactions (e.g., rash; swelling of the face, lips, or tongue), anorexia, nausea, abdominal cramps, visual disturbances, and a bad taste in the mouth. Hyponatremia and water intoxication–induced seizures and coma are also rare, but more common after intranasal use.34 A systematic review suggests that the risk of water intoxication can be minimized with careful monitoring during initiation of desmopressin therapy; supervised administration to minimize the risk of overdose; fluid restriction; prompt assessment for hyponatremia if nausea, vomiting, and headache occur; and oral administration.34,35
Treatment of Secondary and Nonmonosymptomatic Enuresis
If no cause for nocturnal enuresis is evident, primary nocturnal enuresis treatment options are appropriate. Most children with encopresis-associated enuresis and daytime wetting respond to disimpaction, prevention of reaccumulation of stools, and bowel retraining (decreases daytime wetting in 89 percent of patients and nocturnal enuresis in 63 percent of patients).38
A history of snoring, mouth breathing, behavioral problems, and daytime somnolence in patients with enlarged tonsils or adenoids on examination may suggest obstructive sleep apnea. Surgical correction of airway obstruction in these patients improves or cures nocturnal enuresis and daytime wetting.39
Individual psychotherapy, crisis intervention, and family therapy are effective measures for psychogenically induced enuresis.2 Children with dysfunctional voiding have abnormal urine flow patterns on cystometry, a large amount of post-void residual urine, and a normal upper urinary tract on imaging and benefit from a voiding regimen. Biofeedback is effective for motivated children with primary nocturnal enuresis and dysfunctional voiding.25 Biofeedback improves nocturnal enuresis, daytime wetting, constipation, frequency, urgency, voiding patterns, and bladder hyperactivity for up to two years after completion of therapy.25
Children with urge incontinence, normal urine flow, small bladder capacity on urodynamic testing, and normal imaging results benefit from anticholinergic therapy.28 These medications may also be useful in children with both daytime and nocturnal incontinence and in children who do not respond to desmopressin.8,17,28