
Am Fam Physician. 2009;79(4):338-343
Guideline source: American Association for the Study of Liver Diseases
Literature search described? Yes
Evidence rating system used? Yes
Published source:Hepatology, February 2007
Available at:http://www3.interscience.wiley.com/cgi-bin/fulltext/114098985/HTMLSTART
About 350 million persons worldwide have chronic hepatitis B virus (HBV) infection, and 1.25 million persons in the United States are HBV carriers. Updated recommendations from the American Association for the Study of Liver Diseases (AASLD) include the preferred diagnostic, therapeutic, and preventive approaches to chronic HBV.
Definitions and Diagnostic Criteria
Chronic HBV infection is a necroinflammatory disease of the liver caused by persistent infection with HBV, and can be categorized as hepatitis B e antigen (HBeAg) positive or negative. Inactive hepatitis B surface antigen (HBsAg) carriers have HBV infection of the liver without significant, ongoing necroinflammatory disease. HBV infection is resolved when there is no further virologic, biochemical, or histologic evidence of active viral infection or disease. Acute exacerbations or flares include intermittent elevations of transaminase activity to more than 10 times the upper limit of normal and more than twice the baseline value. Reactivation is characterized by the reappearance of active necroinflammatory disease of the liver in persons with inactive HBsAg or resolved hepatitis B. HBeAg clearance is the loss of HBeAg in persons who were previously HBeAg positive. HBeAg seroconversion is defined as the loss of HBeAg and detection of anti-HBeAg in persons who were previously HBeAg positive and anti-HBeAg negative, whereas reversion is the reappearance of HBeAg in persons who were previously HBeAg negative and anti-HBeAg positive.
Diagnostic criteria related to HBV include the following.
Chronic hepatitis B: HBsAg positive for more than six months, serum HBV DNA greater than 20,000 IU per mL (lower values of 2,000 to 20,000 IU per mL often occur with HBeAg-negative chronic hepatitis B), persistent or intermittent elevation in alanine transaminase (ALT) or aspartate transaminase (AST) levels, and liver biopsy showing chronic hepatitis with moderate or severe necroinflammation.
Inactive HBsAg carrier state: HBsAg positive for more than six months, HBeAg negative and anti-HBeAg positive, serum HBV DNA less than 2,000 IU per mL, persistently normal ALT and AST levels, liver biopsy confirming absence of significant hepatitis.
Resolved hepatitis B: known history of acute or chronic hepatitis B or the presence of anti-hepatitis B core antigen (anti-HBcAg) with or without anti-HBsAg, HBsAg negative, undetectable serum HBV DNA (very low levels may be detectable with sensitive prostate-specific antigen assays), and normal ALT levels.
Screening and Prevention
Table 1 lists the high-risk populations that should be screened for HBV infection. Screening includes HBsAg and anti-HBsAg testing. Anti-HBcAg testing may be used, but patients with positive test results should also be screened with HBsAg and anti-HBsAg testing to differentiate infection from immunity or a false-positive result.
| Persons born in areas of high* and intermediate prevalence† rates for HBV, including immigrants and adopted children‡§ |
| Asia (except Sri Lanka) |
| Africa |
| South Pacific Islands |
| Middle East (except Cyprus) |
| European Mediterranean: Greece, Italy, Malta, Portugal, and Spain |
| Arctic (indigenous populations) |
| South America: Argentina, Bolivia, Brazil, Ecuador, Guyana, Suriname, Venezuela, and the Amazon region of Columbia and Peru |
| Independent states of the former Soviet Union |
| Eastern Europe, including Russia, except Hungary |
| Caribbean: Antigua and Barbuda, Dominica, Dominican Republic, Granada, Haiti, Jamaica, Puerto Rico, St. Kitts and Nevis, St. Lucia, St. Vincent and Grenadines, Trinidad and Tobago, and Turks and Caicos |
| Other high-risk groups recommended for screening |
| Household and sexual contacts of HBsAg-positive persons§ |
| Persons who have ever injected drugs§ |
| Persons with multiple sex partners or a history of sexually transmitted disease* |
| Men who have sex with men§ |
| Inmates of correctional facilities§ |
| Persons with chronically elevated alanine transaminase or aspartate transaminase levels§ |
| Persons infected with hepatitis C virus or human immunodeficiency virus§ |
| Patients undergoing renal dialysis§ |
| All pregnant women |
HBV is transmitted by perinatal, percutaneous, and sexual exposure. HBsAg carriers should be counseled about the risks of transmitting the infection to others (Table 2).
| Persons who are HBsAg-positive |
| Advise sexual contacts to be vaccinated |
| Use barrier protection during sexual intercourse if partner is not vaccinated or naturally immune |
| Do not share toothbrushes or razors |
| Cover open cuts and scratches |
| Clean blood spills with detergent or bleach |
| Do not donate blood, organs, or sperm |
| Children and adults who are HBsAg-positive |
| Can participate in all activities, including contact sports |
| Should not be excluded from daycare or school participation, and should not be isolated from other children |
| Can share food and utensils and kiss others |
Some high-risk persons, such as household and sexual contacts of HBsAg-positive persons, who test negative for HBV seromarkers should receive HBV vaccination. Newborns of mothers with HBV infection should receive HBV and hepatitis B immune globulin vaccination. Follow-up testing to detect postvaccination response should be performed in persons who remain at risk of infection (e.g., infants of mothers who are carriers, health care workers, patients undergoing dialysis, sexual contacts of carriers).
Evaluation
The evaluation of patients with chronic HBV infection (Table 3) includes a patient history and physical examination. Laboratory testing should also be performed in some patients.
| Initial evaluation |
| History and physical examination |
| Family history of liver disease, HCC |
| Laboratory tests to assess liver disease (complete blood count with platelets, hepatic panel, and prothrombin time) |
| Tests for HBV replication (HBeAg or anti-HBeAg, HBV DNA) |
| Tests to rule out viral coinfections (anti-HCV, anti-HDV in persons from countries where HDV infection is common and in those with a history of injection drug use), and anti-HIV in those at risk |
| Tests to screen for HCC (α-fetoprotein levels at baseline and, in high-risk patients, ultrasonography) |
| Consider liver biopsy to grade and stage liver disease for patients who meet criteria for chronic hepatitis |
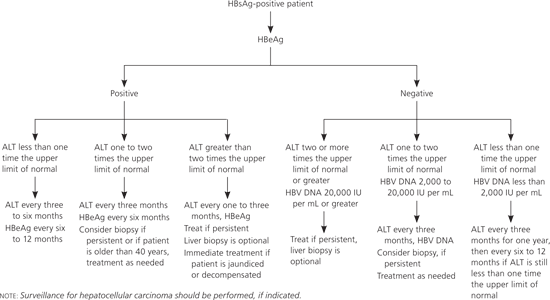
| Suggested follow-up for patients not considered for treatment (HBeAg positive, HBV DNA greater than 20,000 IU per mL, and normal ALT level) |
| ALT levels every three to six months, more often if ALT level becomes elevated |
| If ALT levels are between one and two times the upper limit of normal, recheck ALT every one to three months; consider liver biopsy if patient is older than 40 years, ALT level is borderline or mildly elevated on serial tests; consider treatment if biopsy shows moderate or severe inflammation or significant fibrosis |
| If ALT is greater than two times the upper limit of normal for three to six months, the patient is HBeAg positive, and HBV DNA is greater than 20,000 IU per mL, consider liver biopsy and treatment |
| Consider screening for HCC in relevant populations |
| Inactive HBsAg carrier state |
| ALT levels every three months for one year; if persistently normal, ALT levels every six to 12 months |
| If ALT level is greater than one to two times the upper limit of normal, check serum HBV DNA level and exclude other causes of liver disease; consider liver biopsy if ALT level is borderline or mildly elevated on serial tests or if HBV DNA levels are persistently greater than 20,000 IU per mL; consider treatment if biopsy shows moderate or severe inflammation or significant fibrosis |
| Consider screening for HCC in relevant populations |
HBV DNA assays are important in the evaluation of patients with chronic HBV infection, and serial monitoring can help determine prognosis and need for treatment. Liver biopsy can assess liver damage and rule out other causes of liver disease. Patients not initially considered for treatment should receive follow-up testing (Figure 1).
Treatment
The main goal of treatment is preventing cirrhosis, hepatic failure, and hepatocellular carcinoma. Other goals include sustained suppression of HBV replication and remission of liver disease. HBeAg-positive and HBeAg-negative patients who meet criteria for chronic HBV infection should be evaluated for treatment, especially those with persistently elevated ALT levels.
Interferons are used for a predefined duration, whereas nucleoside analogue (NA) treatment is usually used until specific endpoints are achieved. Antiviral resistance is a major concern with long-term NA treatment. Among the therapies, lamivudine is associated with the highest and entecavir with the lowest resistance in NA-naïve patients. Patients with minimal disease or who are unlikely to achieve sustained response should not be treated with NA, especially those who are younger than 30 years. The most potent NA with the lowest resistance should be used and compliance reinforced.
Combination therapy has been beneficial for human immunodeficiency virus and hepatitis C virus infections. However, it has not been shown to be superior to monotherapy in the treatment of HBV infection.