Am Fam Physician. 2010;82(9):1087-1095
Patient information: See related handout on influenza, written by the authors of this article.
Author disclosure: Nothing to disclose.
Influenza is a contagious airborne viral illness characterized by abrupt onset of symptoms. Fever, myalgia, headache, rhinitis, sore throat, and cough are commonly reported symptoms. The diagnosis should be made clinically, and the decision to begin antiviral therapy should not be delayed for laboratory confirmation of influenza. The 2009 pandemic influenza A (H1N1) virus is expected to continue to circulate during the 2010–2011 season, but it is not certain whether it will replace or cocirculate with seasonal influenza A subtypes that have been circulating since 1977. The 2009 H1N1 virus is largely resistant to adamantanes, but it is sensitive to neuraminidase inhibitors such as oseltamivir. Neuraminidase inhibitors have modest effectiveness in reducing influenza-related symptoms in patients at low risk of complications. Patients at high risk of complications, including pregnant women, should be treated with antiviral agents, preferably within 48 hours of symptom onset. Family physicians should follow guidelines from the World Health Organization and the Centers for Disease Control and Prevention when treating patients with influenza or influenza-like symptoms.
Influenza is a highly contagious airborne viral illness. The virus enters the respiratory tract cells of the host and, if not neutralized by antibodies, begins proliferating.1 Systemic symptoms, such as fever, myalgia, headache, malaise, rhinitis, sore throat, and cough, are thought to result from the release of inflammatory mediators in response to viral activity.1 The incubation period is 18 to 72 hours, but viral shedding may occur up to 24 hours before symptom onset and continue for five to 10 days.1,2 Influenza is typically uncomplicated and self-limited in otherwise healthy patients. However, severe complications, such as pneumonia, encephalitis, respiratory failure, multiorgan failure, and death, can occur.3,4 According to estimates from the World Health Organization (WHO), 3 to 5 million cases of severe influenza-related illness and 250,000 to 500,000 influenza-related deaths occur worldwide every year.5
| Clinical recommendation | Evidence rating | References |
|---|---|---|
| The decision to begin antiviral treatment should be based on the clinical diagnosis of influenza, not on test results. | C | 4, 16, 20, 21 |
| Patients at risk of complications from influenza should begin antiviral treatment within 48 hours of symptom onset. | C | 3, 4, 8, 16, 20, 21 |
| The choice of antiviral agent should be based on local patterns of virus activity and susceptibility. | C | 3, 4, 8, 16, 20, 21 |
Influenza viruses are single-stranded RNA viruses belonging to the Orthomyxovirus family. They can mutate quickly, which makes development of effective vaccines and antiviral agents a challenge.6 Influenza A viruses cause most outbreaks and are more pathogenic than other types of influenza viruses.1,3,7 In the spring of 2009, a triple-reassortant strain of influenza A (H1N1) that contained swine, avian, and human genes caused several outbreaks in Mexico and the United States; new cases were soon reported worldwide.2,4,8–12 On June 11, 2009, WHO declared a global pandemic based on 30,000 cases of H1N1 influenza in 74 countries.9 The Centers for Disease Control and Prevention (CDC) estimates that by April 19, 2010, about 61 million persons in the United States had been infected with 2009 H1N1 influenza, 274,000 had been hospitalized, and 12,470 had died.13 WHO declared an end to the pandemic on August 10, 2010.14
Typically, persons at risk of influenza-related complications include older adults, young children, persons with chronic medical conditions, and pregnant women3,4,8 (Table 14,8,15,16 ). However, during the 2009–2010 influenza season, about one third of patients with complicated influenza who were admitted to intensive care units were previously healthy and had no identifiable risk factors.3,8

| Persons with coexisting medical conditions |
| Any condition that may compromise the handling of respiratory secretions (e.g., neuromuscular diseases, cerebral palsy, stroke, seizure disorder, dementia) |
| Asthma or other chronic pulmonary disease |
| Chronic liver disease |
| Chronic renal disease |
| Heart disease (acquired or congenital) |
| Immunosuppression (e.g., human immunodeficiency virus infection, cancer, transplant recipients, use of immunosuppressive medications) |
| Long-term aspirin therapy in patients younger than 19 years |
| Metabolic disorders (acquired [e.g., diabetes mellitus] or inherited [e.g., mitochondrial disorders]) |
| Morbid obesity |
| Sickle cell anemia and other hemoglobinopathies |
| Special groups |
| Adults 65 years and older |
| Children younger than 5 years (particularly those younger than 2 years) |
| Institutionalized adults (e.g., those living in long-term care facilities, prisons, or college dormitories) |
| Pregnant and postpartum women (up to 2 weeks postpartum, including pregnancy loss) |
Influenza caused by 2009 H1N1 is expected to continue during the 2010–2011 influenza season and in the future, but it is not known whether this virus will replace or cocirculate with one or more of the two seasonal influenza A viruses (seasonal H1N1 and H3N2) that have circulated since 1977. The CDC Web site (http://www.cdc.gov/flu) provides regular updates on influenza activity and resistance patterns in the United States. Local public health authorities provide similar information, which can help physicians make treatment decisions for patients with suspected influenza.
Prevention
The CDC recommends that all persons six months and older receive influenza vaccination this season.17,18 The trivalent (seasonal) vaccine will protect against 2009 H1N1 influenza and contains the same strain used in the 2009–2010 monovalent H1N1 vaccine, plus an H3N2 virus and influenza B virus.17,18 A complete discussion of the different influenza vaccine types is available on the CDC Web site (http://www.cdc.gov/flu/professionals/acip/index.htm).17
Treatment of Patients with Influenza-Like Illness
When influenza cases are reported in the community, most patients presenting with symptoms suggestive of uncomplicated influenza should be diagnosed clinically.3 Patients should be instructed to return for follow-up if their symptoms worsen or do not improve within 72 hours.3 During the 2009 H1N1 pandemic, prompt empiric antiviral treatment was recommended for certain high-risk groups (children younger than two years, adults 65 years and older, pregnant women, and persons with certain medical conditions; see Table 14,8,15,16).3,15 All patients with signs of severe, progressive illness should be promptly referred for hospitalization3,16 (Table 23 ). Telephone triage of persons with influenza-like symptoms helps prevent the exposure of these patients to other patients in waiting rooms.19
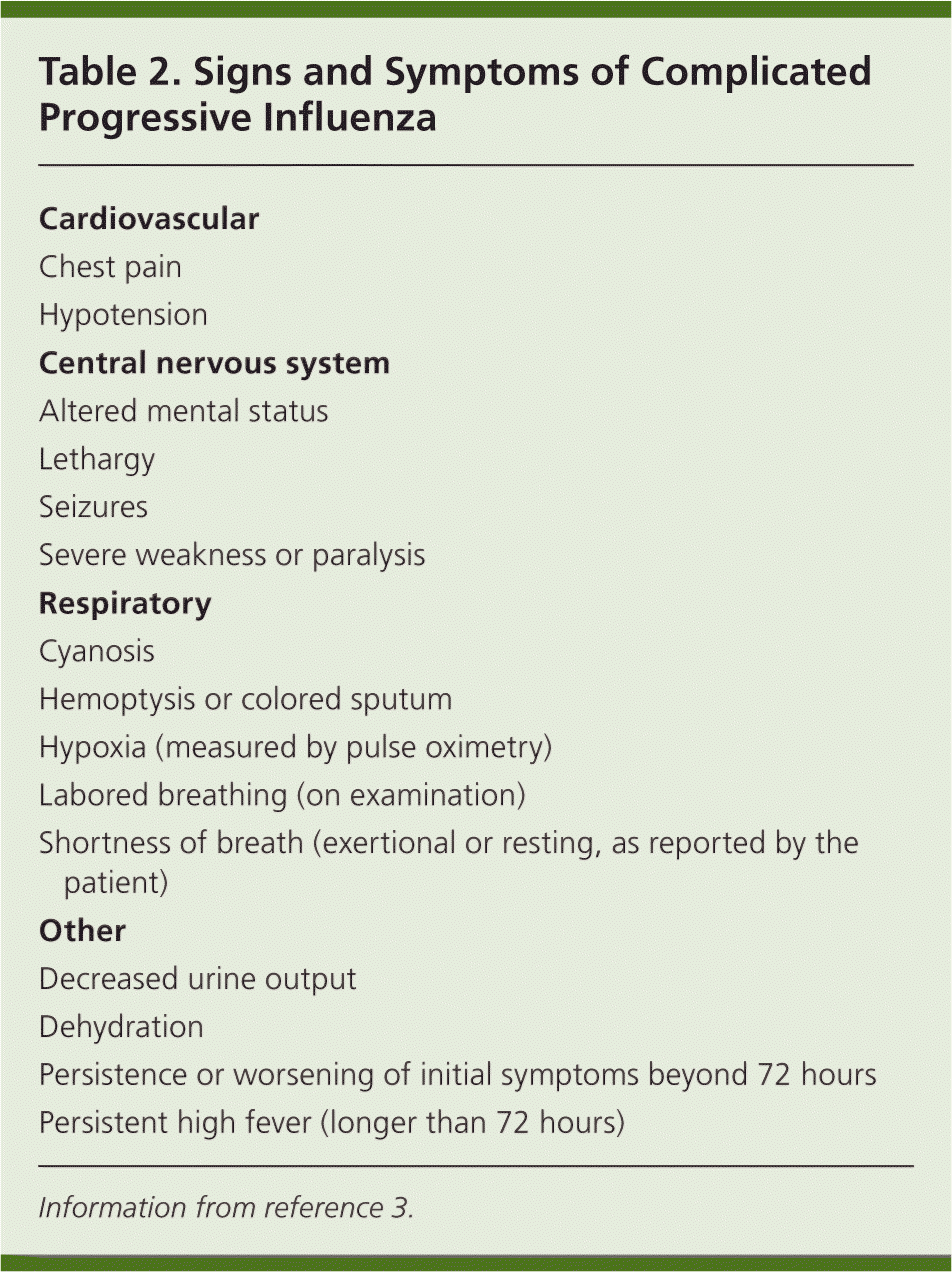
| Cardiovascular |
| Chest pain |
| Hypotension |
| Central nervous system |
| Altered mental status |
| Lethargy |
| Seizures |
| Severe weakness or paralysis |
| Respiratory |
| Cyanosis |
| Hemoptysis or colored sputum |
| Hypoxia (measured by pulse oximetry) |
| Labored breathing (on examination) |
| Shortness of breath (exertional or resting, as reported by the patient) |
| Other |
| Decreased urine output |
| Dehydration |
| Persistence or worsening of initial symptoms beyond 72 hours |
| Persistent high fever (longer than 72 hours) |
Diagnosis
The CDC, WHO, and the Infectious Diseases Society of America recommend that physicians diagnose influenza clinically and perform testing only in the following groups: hospitalized patients with influenza-like illness; patients who died of an influenza-like illness (to clarify etiology); and patients for whom decisions about infection control and treatment of close contacts is a concern (e.g., child care and health care workers, nursing home residents).3,4,16,20,21 Several diagnostic tests for influenza are available (Table 3).4,20,21 Rapid influenza diagnostic tests have variable sensitivities (10 to 70 percent); therefore negative results do not rule out influenza.4,21 The specificities of rapid tests are generally high (greater than 95 percent).4,21 Although many physicians use rapid influenza tests, clinical judgment should prevail, especially in view of the limitations of such tests.20,21 Real-time reverse transcriptase polymerase chain reaction tests and viral cultures have much higher sensitivities than rapid tests, but results generally take more than 24 hours. Both are definitive when diagnostic testing for influenza is indicated.3,4,20
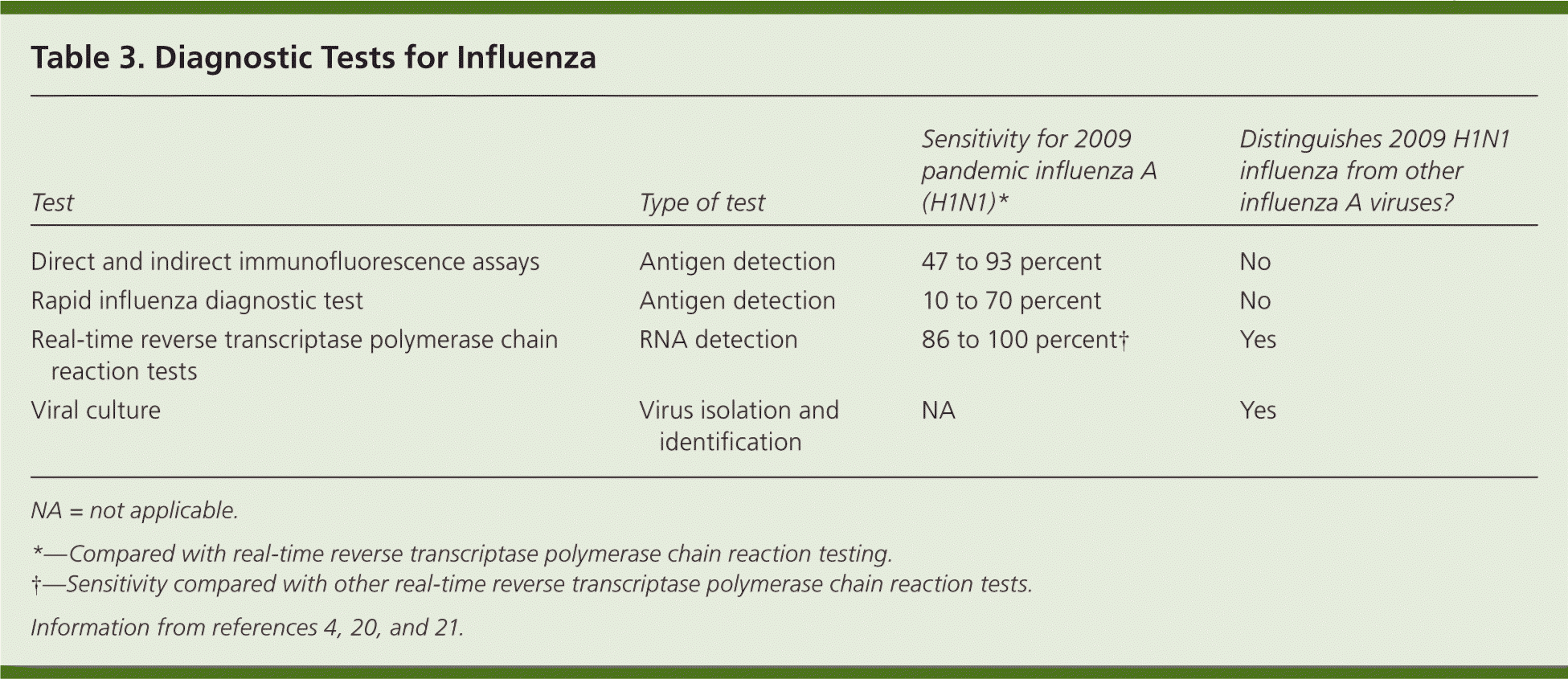
| Test | Type of test | Sensitivity for 2009 pandemic influenza A (H1N1)* | Distinguishes 2009 H1N1 influenza from other influenza A viruses? |
|---|---|---|---|
| Direct and indirect immunofluorescence assays | Antigen detection | 47 to 93 percent | No |
| Rapid influenza diagnostic test | Antigen detection | 10 to 70 percent | No |
| Real-time reverse transcriptase polymerase chain reaction tests | RNA detection | 86 to 100 percent† | Yes |
| Viral culture | Virus isolation and identification | NA | Yes |
Antiviral Therapy
Treatment should not be delayed until the results of diagnostic tests are available.3,4,20,21 The CDC recommends initiating treatment as soon as possible after the onset of influenza-like symptoms in patients meeting certain criteria15 (Table 43,4,8,15,16,20–23). The CDC also recommends antiviral prophylaxis for persons who have had close contact with an infected person during the infectious period.15

| Consider antiviral chemoprophylaxis if close contact* has occurred with an infected person during the infectious period† |
| Health care workers |
| Persons at risk of complications from influenza |
| Pregnant women |
| Do not prescribe antiviral chemoprophylaxis |
| Healthy children and adults |
| Persons who had close contact* with an infected person outside the infectious period |
| Persons whose last close contact* with an infected person was more than 48 hours before presentation |
| Prescribe antiviral treatment |
| Hospitalized patients with severe, complicated influenza-like illness (see Table 2) or laboratory-confirmed influenza |
| Outpatients with influenza-like illness or laboratory-confirmed influenza who are at risk of complications (see Table 1) |
| Outpatients with severe, complicated influenza-like illness (see Table 2) or laboratory-confirmed influenza‡ |
MANAGEMENT OF INFLUENZA DURING PREGNANCY
Pregnant and postpartum women (including those who have had pregnancy loss) are at risk of severe influenza-related complications because of the changes in immune, respiratory, and cardiovascular systems that occur during pregnancy.3,24–27 The CDC recommends that neuraminidase inhibitors be prescribed for pregnant women and for those up to two weeks postpartum who have suspected or confirmed influenza.26 Women can continue to breastfeed while being treated with antivirals.14
ANTIVIRAL AGENTS
Neuraminidase Inhibitors. Neuraminidase inhibitors block the viral neuraminidase enzyme, which is critical in releasing virions from the infected host's cells.28 These drugs are active against influenza A and B.6,7,29 As of April 2010, more than 99 percent of the 2009 H1N1 viruses were susceptible to oseltamivir (Tamiflu) and zanamivir (Relenza).15,29 None of the H3N2 or influenza B viruses tested were resistant to oseltamivir.17
| Drug/available formulations | Dosage | FDA-approved indications | FDA pregnancy category and contraindications | Possible adverse effects | ||
|---|---|---|---|---|---|---|
| Oseltamivir (Tamiflu) | Children 1 to 12 years Prophylaxis | Influenza prophylaxis in patients 1 year and older Treatment of uncomplicated acute influenza in patients 1 year and older who have been symptomatic for no more than 2 days | Category C (preferred treatment in pregnancy) Contraindicated in patients with known serious hypersensitivity to oseltamivir or any component of the product | Nausea, vomiting, allergic reactions (rash, facial swelling) Rarely, serious skin reactions (toxic epidermal necrolysis, Stevens-Johnson syndrome, and erythema multiforme) and transient neuropsychiatric events(self-injury or delirium; causal relationship has not been established) | ||
| Capsules (30, 45, and 75 mg) Intravenous form (in clinical trials) Powder for oral suspension | ≤ 33 lb (≤ 15 kg): 30 mg once daily for 10 days* > 33 to 51 lb (> 15 to 23 kg): 45 mg once daily for 10 days* > 51 to 88 lb (> 23 to 40 kg): 60 mg once daily for 10 days* > 88 lb (> 40 kg): adult dosage | |||||
| Treatment | ||||||
| ≤ 33 lb (= 15 kg): 30 mg twice daily for 5 days | ||||||
| > 33 to 51 lb (> 15 to 23 kg): 45 mg twice daily for 5 days | ||||||
| > 51 to 88 lb (> 23 to 40 kg): 60 mg twice daily for 5 days | ||||||
| > 88 lb (> 40 kg): adult dosage | ||||||
| Adults and children 13 years and older | ||||||
| Prophylaxis | ||||||
| 75 mg once daily for at least 10 days* | ||||||
| Patients with impaired renal function: 75 mg every other day for at least 10 days* | ||||||
| Treatment | ||||||
| 75 mg twice daily for 5 days | ||||||
| Patients with impaired renal function: 75 mg once daily for5 days | ||||||
| Zanamivir (Relenza) | Children | Influenza prophylaxis in patients 5 years and older Treatment of influenza in patients 7 years and older who have been symptomatic for no more than 2 days | Category C Contraindicated in patients with milk allergy or history of allergic reaction to zanamivir or any component of the product Not recommended in patients with underlying reactive airways disease (e.g., asthma, chronic obstructive pulmonary disease); may worsen pulmonary status in these patients | Cough, nasal and throat discomfort, bronchospasm, worsening of pulmonary status, allergic reactions(including anaphylaxis) | ||
| Intravenous form (not FDA approved) Powder for inhalation, supplied in blister packs of 10 5-mg doses and packaged with a Diskhaler (should not be used with any other inhalation device) | Prophylaxis | |||||
| 5 years and older: adult dosage | ||||||
| Treatment | ||||||
| 7 years and older: adult dosage | ||||||
| Adults | ||||||
| Prophylaxis | ||||||
| 10 mg (2 5-mg inhalations) once daily for 10 days | ||||||
| Treatment | ||||||
| 10 mg (2 5-mg inhalations) twice daily for 5 days | ||||||
| Peramivir‡ | NA | NA | NA | Diarrhea, nausea, vomiting, neutropenia | ||
| Intravenous form (single-use vials) | ||||||
| Amantadine | Children | Prophylaxis and treatment of influenza A virus infection in patients 1 year and older Treatment of parkinsonism and drug-induced extrapyramidal reactions | Category C Contraindicated in patients with known hypersensitivity to amantadine or rimantadine | Central nervous system effects (e.g., insomnia, lightheadedness, difficulty concentrating, delirium, seizures, hallucinations) Suicidal ideation has been reported (more common in patients taking other agents with central nervous system effects) | ||
| Capsules and tablets (100 mg) Oral solution | Prophylaxis | |||||
| 1 to 9 years of age: 5 mg per kg per day (up to 150 mg per day) in 2 divided doses for at least 10 days after exposure§ | ||||||
| 10 years and older (≥ 88 lb [≥ 40 kg]): adult dosage | ||||||
| Treatment | ||||||
| 1 to 9 years of age: 5 mg per kg per day (up to 150 mg per day) in 2 divided doses for 5 days | ||||||
| 10 years and older (≥ 88 lb [≥ 40 kg]): adult dosage | ||||||
| Adults | ||||||
| Prophylaxis | ||||||
| 100 mg twice daily for at least 10 days after exposure§ | ||||||
| Creatinine clearance less than 50 mL per minute: 100 mg once daily for 10 days after exposure | ||||||
| Treatment | ||||||
| 100 mg twice daily for 5 days | ||||||
| 65 years and older: 100 mg once daily for 5 days | ||||||
| Creatinine clearance less than 50 mL per minute: 100 mg once daily for 5 days | ||||||
| Rimantadine (Flumadine) Tablets (100 mg) | Children | Influenza A virus prophylaxis in children 1 to 16 years of age Prophylaxis and treatment of influenza A virus infection in adults and adolescents 17 years and older | Category C Contraindicated in patients with known hypersensitivity to amantadine or rimantadine | Similar to amantadine, but less common | ||
| Prophylaxis∥ | ||||||
| 1 to 9 years of age: 5 mg per kg per day (up to 150 mg per day) in 2 divided doses for 5 to 7 days after exposure | ||||||
| 10 years and older: adult dosage | ||||||
| Adults | ||||||
| Prophylaxis∥ | ||||||
| 100 mg twice daily for 5 to 7 days after exposure | ||||||
| 65 years and older: 100 mg once daily for 5 to 7 days after exposure | ||||||
| Treatment | ||||||
| 100 mg twice daily for 5 days | ||||||
| 65 years and older: 100 mg once daily for 5 days | ||||||
| Ribavirin | As specified in an approved research protocol | Approved for use only in patients with respiratory syncytial virus infection and in those with hepatitis C (in combination with other agents), but has been used in hospitalized immunocompromised patients with influenza Should be used only in critical patients and on a case-by-case basis as part of an approved research protocol | Category X See package insert for contraindications and FDA boxed warnings | Cardiovascular events, arrhythmias, hematologic derangements, seizures, asthenia, worsening of pulmonary status | ||
| Intravenous form (available through the FDA as part of an approved research protocol) | ||||||
| Powder for nebulizer solution (Virazole) | ||||||
| Capsules and tablets (for treatment of hepatitis C) | ||||||
Zanamivir is an oral inhalation agent that is approved for influenza prophylaxis in patients five years and older, and for treatment of influenza in patients seven years and older who have been symptomatic for no more than two days.23 Inhaled zanamivir can worsen pulmonary status in patients with underlying pulmonary disorders, and should not be used in these patients.23,29 Relenza is a mixture of zanamivir and lactose drug carrier; therefore milk allergy is a contraindication.23
The effectiveness of oseltamivir and zanamivir in outpatient populations was evaluated in a 2009 systematic review that included trials involving healthy adults and trials involving persons at risk of influenza-related complications.33 Oseltamivir reduced the duration of symptoms by 0.55 days among healthy adults (1,410 participants) and by 0.74 days in persons at risk of complications (1,472 participants). For zanamivir, time to symptom alleviation was reduced by 0.57 days in healthy adults (2,701 participants) and by 0.98 days in persons at risk of complications (1,252 participants).33 A 2010 Cochrane review concluded that inconsistencies in the available studies, as well as unavailability of data from other studies, preclude any firm conclusions to be made about the effectiveness of oseltamivir in preventing influenza-related complications in healthy adults, and that its effectiveness in reducing influenza-related symptoms is modest.34,35 A 2009 systematic review and meta-analysis of oseltamivir and zanamivir use in children concluded that treatment of influenza with either agent produced faster resolution of symptoms by 0.5 to 1.5 days.36 Most of the data about the use of neuraminidase inhibitors in outpatient settings was derived from studies of seasonal influenza and influenza-like illnesses. Whether these conclusions can be extrapolated to patients with 2009 H1N1 influenza is not clear.16,34
In hospitalized patients, early treatment with oseltamivir is associated with increased survival rates in critically ill patients with 2009 H1N1 influenza.10,12,25 Reduced dosages are recommended for patients with impaired renal function.22,29 No dosage adjustment is needed for patients with renal disease who are taking zanamivir.23
Neuropsychiatric adverse events (self-injury and delirium) have been reported in adolescents taking oseltamivir, but these events are rare (one per 100,000 prescriptions).15,29,37,38 It is not clear whether these events are associated with oseltamivir use, influenza infection, or some other factor. Similar events have been reported in adults taking oseltamivir.39
Peramivir is a neuraminidase inhibitor formulated for intravenous administration.29,40 It is an investigational product and is currently being evaluated in clinical trials.8 In October 2009, the U.S. Food and Drug Administration issued an emergency use authorization allowing peramivir to be used in the treatment of hospitalized patients with H1N1 influenza; this authorization expired in June 2010.15
Adamantanes. Adamantanes are M2 ion channel blockers; they interfere with hydrogen ion channel activity of the influenza A virus, thus blocking its entry into the host cells. Adamantanes are active only against influenza A viruses.6,7,28,29 During the 2009–2010 influenza season, more than 99 percent of circulating influenza viruses in the United States were H1N1 viruses that were resistant to adamantanes.15 However, surveillance data from the CDC show that isolates resistant to oseltamivir may remain susceptible to amantadine, rimantadine (Flumadine), and zanamivir.15 Although adamantanes currently have no role in the management of influenza, emerging resistance patterns may necessitate re-evaluation of their use.15,16
Ribavirin. Ribavirin, an antiviral drug with in vitro activity against DNA and RNA viruses, is approved for the treatment of respiratory syncytial virus infection and for the treatment of hepatitis C in combination with other agents.6,41 It has been used to treat critically ill patients with influenza, although data are limited.6,42,43 WHO guidelines recommend that ribavirin be used to treat influenza only as part of an approved research protocol.16
Additional Considerations
Over-the-counter antipyretics and anti-inflammatory agents can be used for symptomatic relief in patients with influenza; aspirin or aspirin-containing medications should not be used in children because of the risk of Reye syndrome.16 Bacterial coinfection or development of complications may necessitate the use of antibiotics in patients with influenza.16
Many patients with influenza-like illnesses use supplements such as homeopathic Oscillococcinum, vitamin C, zinc, echinacea, elderberry, garlic, ginseng, and olive leaf to alleviate symptoms.7,44–47 Most clinical trials of complementary and alternative medicine in influenza-like illnesses have methodologic flaws that prevent conclusions to be made about the effectiveness of these preparations.