
Am Fam Physician. 2019;100(12):751-758
Related Practice Guideline: Influenza Vaccination: Updated Recommendations from ACIP
Author disclosure: No relevant financial affiliations.
Influenza is an acute viral respiratory infection that causes significant morbidity and mortality worldwide. Three types of influenza cause disease in humans. Influenza A is the type most responsible for causing pandemics because of its high susceptibility to antigenic variation. Influenza is highly contagious, and the hallmark of infection is abrupt onset of fever, cough, chills or sweats, myalgias, and malaise. For most patients in the outpatient setting, the diagnosis is made clinically, and laboratory confirmation is not necessary. Laboratory testing may be useful in hospitalized patients with suspected influenza and in patients for whom a confirmed diagnosis will change treatment decisions. Rapid molecular assays are the preferred diagnostic tests because they can be done at the point of care, are highly accurate, and have fast results. Treatment with one of four approved anti-influenza drugs may be considered if the patient presents within 48 hours of symptom onset. The benefit of treatment is greatest when antiviral therapy is started within 24 hours of symptom onset. These drugs decrease the duration of illness by about 24 hours in otherwise healthy patients and may decrease the risk of serious complications. No anti-influenza drug has been proven superior. Annual influenza vaccination is recommended for all people six months and older who do not have contraindications.
Influenza is an acute respiratory infection caused by a negative-strand RNA virus of the Orthomyxoviridae family. There are three distinct types of influenza viruses that infect humans: influenza A, B, and C. Influenza A infects multiple species, including humans, swine, equines, and birds. It is more susceptible to antigenic variation and, hence, is the cause of major pandemics.1,2 The surface of the virion envelope is covered with proteins hemagglutinin (HA), neuraminidase (NA), and matrix 2.1 Antigenic variation is associated with changes in the HA or NA surface proteins and is generally classified as antigenic drift or shift.1 Antigenic drift involves small, gradual amino acid substitutions of the HA or NA proteins that can result in smaller outbreaks. Antigenic shift occurs when there are significant changes in the HA or NA proteins that create novel influenza subtypes with the potential to cause widespread pandemics.1,3 Each influenza A subtype is characterized by numbering both the HA and NA proteins (e.g., H3N2, H5N1).

| Recommendation | Sponsoring organization |
|---|---|
| Do not routinely avoid influenza vaccination in egg-allergic patients. | American Academy of Allergy, Asthma, and Immunology |
Epidemiology
From 2010 to 2018, estimated seasonal influenza activity in the United States ranged from 9.3 million to 49 million cases and 12,000 to 79,000 deaths per year.4 The 2017–2018 influenza season was the third most severe since 2003–2004 and was characterized by high severity of disease in all age groups.5 Most cases were secondary to the A/H3N2 subtype, and low vaccine effectiveness was a significant contributing factor.5 Based on public health laboratory specimens, the predominant influenza A subtype for the 2018–2019 season was (H1N1)pdm09 (56.6% of positive specimens), followed by the A/H3N2 subtype (43.6%) and influenza B (4%).6
Seasonal influenza occurs primarily in the colder months in temperate regions. The primary mode of transmission is via inhalation of infectious respiratory particles (large droplet transmission) when an infected person coughs or sneezes. There is also evidence of airborne (small particles transmitted by talking or exhalation) and fomite transmission.6,7 The typical incubation period is 24 to 48 hours. Patients are infectious one to two days before symptom onset and for five to seven days afterward. Children and immunosuppressed people may exhibit prolonged viral shedding.8,9
Since 2011, outbreaks of a swine-origin H3N2 virus have been reported in the United States. Cases predominantly involved young children exposed to swine, often at agricultural fairs.12,13 Additionally, the H5N1 avian influenza virus has become a global concern. First identified solely in wild geese populations in China in 1997, the virus quickly spread to domestic poultry and ultimately to human populations. There are now avian influenza cases reported worldwide, although poultry-to-human and human-to-human transmission remains relatively low. Despite low transmissibility, the reported fatality rate is high (approximately 60%).14
Prevention
The Centers for Disease Control and Prevention's (CDC's) Advisory Committee on Immunization Practices (ACIP) and the American Academy of Family Physicians (AAFP) recommend annual influenza vaccination for all people six months and older who do not have contraindications.15,16 Vaccination efforts should target people at increased risk of complicated or severe influenza (Table 117–19) and those who care for or live with high-risk individuals, including health care professionals.15 Two previous FPM articles provided communication strategies and tools for increasing influenza vaccination rates in practice.20,21

| People with coexisting medical conditions |
| Any condition that may compromise the handling of respiratory secretions (e.g., neuromuscular diseases, cerebral palsy, stroke, seizure disorder, dementia) |
| Asthma or other chronic pulmonary disease |
| Chronic kidney disease |
| Chronic liver disease |
| Heart disease (acquired or congenital) |
| Immunosuppression (e.g., HIV infection, cancer, transplant recipients, use of immunosuppressive medications) |
| Long-term aspirin therapy in patients younger than 19 years |
| Metabolic disorders (acquired [e.g., diabetes mellitus] or inherited [e.g., mitochondrial disorders]) |
| Morbid obesity |
| Sickle cell anemia and other hemoglobinopathies |
| Special groups |
| Adults 65 years and older |
| American Indians and Alaska Natives |
| Children younger than 5 years (particularly those younger than 2 years) |
| Institutionalized adults (e.g., residents of nursing homes or chronic care facilities) |
| Pregnant and postpartum women (up to 2 weeks postpartum, including pregnancy loss) |
Multiple formulations of the influenza vaccine are available, including inactivated influenza vaccines (IIV); a recombinant inactivated vaccine (RIV); and a live, attenuated influenza vaccine (LAIV). For physicians and patients concerned about egg allergy, quadrivalent RIV and cell culture–based quadrivalent IIV are completely egg-free.15
An age-appropriate IIV or RIV is suitable for most patients; LAIV is contraindicated in pregnant women, immunosuppressed people, children younger than two years, children two to four years of age who have had asthma or wheezing in the past 12 months, and adults 50 years and older.15 For children six months to eight years of age, two doses of IIV administered at least four weeks apart are recommended if the child has not received at least two doses of the influenza vaccine previously. RIV is not licensed for children younger than 18 years.15
In August 2019, ACIP recommended that LAIV may be used in age-appropriate patients without contraindications for the 2019–2020 influenza season because it was deemed as effective as the IIV for prevention of the H3N2 and influenza B viruses.15,22 Notably, influenza-specific antiviral medications may reduce the effectiveness of LAIV if given within two days before to 14 days after vaccine administration.15 The AAFP has made a preferential recommendation for vaccination with IIV over LAIV.16
A high-dose, inactivated trivalent vaccine (Fluzone High-Dose) is licensed for adults 65 years and older, and multiple studies show superior effectiveness compared with the standard-dose trivalent IIV (number needed to treat to prevent one additional case of laboratory-confirmed influenza = 220 compared with standard-dose vaccine).23,24 An adjuvanted inactivated trivalent vaccine (Fluad) is also licensed for use in adults 65 years and older.15
Clinical Manifestations and Complications
Influenza most often causes an uncomplicated respiratory infection with cough, fever, myalgias, chills or sweats, and malaise that persists for two to eight days. Onset is typically rapid. Atypical gastrointestinal symptoms such as vomiting and diarrhea can occur in children. A minority of patients, especially older adults, young children, and those with medical comorbidities, will experience severe disease due to viral or secondary bacterial pneumonia with respiratory and multiorgan failure.8,25 Extrapulmonary complications are rare (Table 2).8,19,25–27
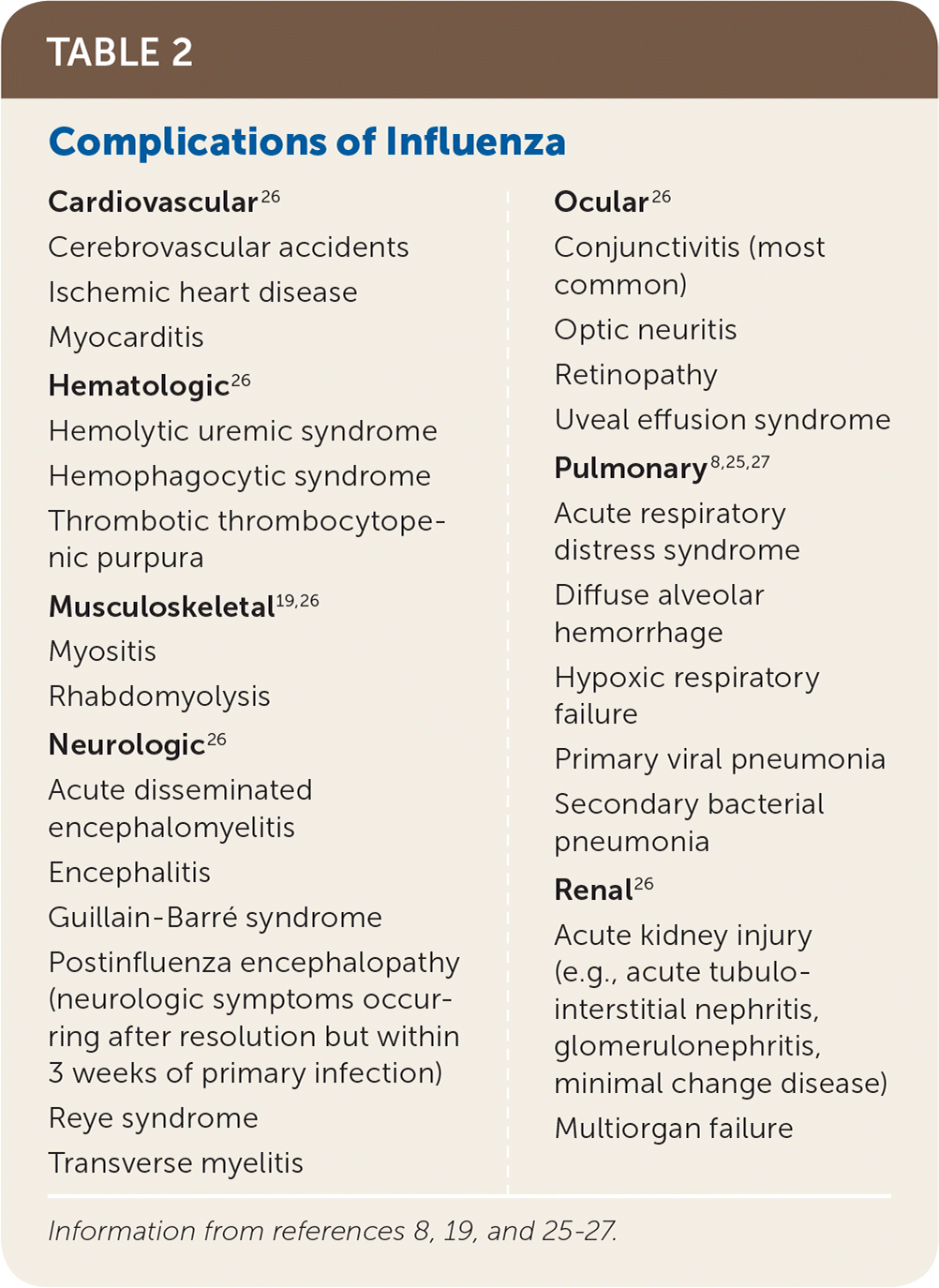
| Cardiovascular26 Cerebrovascular accidents Ischemic heart disease Myocarditis Hematologic26 Hemolytic uremic syndrome Hemophagocytic syndrome Thrombotic thrombocytopenic purpura Musculoskeletal19,26 Myositis Rhabdomyolysis Neurologic26 Acute disseminated encephalomyelitis Encephalitis Guillain-Barré syndrome Postinfluenza encephalopathy (neurologic symptoms occurring after resolution but within 3 weeks of primary infection) Reye syndrome Transverse myelitis | Ocular26 Conjunctivitis (most common) Optic neuritis Retinopathy Uveal effusion syndrome Pulmonary8,25,27 Acute respiratory distress syndrome Diffuse alveolar hemorrhage Hypoxic respiratory failure Primary viral pneumonia Secondary bacterial pneumonia Renal26 Acute kidney injury (e.g., acute tubulo-interstitial nephritis, glomerulonephritis, minimal change disease) Multiorgan failure |
History and Physical Examination
In outpatient and emergency department settings, testing for influenza virus is not necessary to start antiviral treatment in a patient with suspected influenza infection, especially during seasons when influenza A and B viruses are circulating in the local community.19,28 Rather, the diagnosis is made clinically based on presenting signs and symptoms, or if the patient has a suspected influenza-associated complication, such as exacerbation of a chronic disease, concomitant pneumonia, or rhabdomyolysis.19,28
A symptom-only clinical prediction rule may aid clinicians in diagnosing influenza.29 It assigns 2 points for fever and cough, 2 points for myalgias, 1 point for chills or sweats, and 1 point for symptom onset within the past 48 hours. Patients with 2 or fewer points are at low risk of influenza, whereas those with 4 or more points are at high risk and may be considered for empiric treatment.29
Diagnostic Testing
According to the CDC, influenza testing can be considered when the results will modify management or when a patient with signs or symptoms of influenza is hospitalized.19 Similarly, the Infectious Diseases Society of America (IDSA) suggests testing if the results will curtail the use of unnecessary antibiotics or laboratory testing, or result in prophylactic treatment of high-risk household contacts.28 A prospective study performed at a university health clinic found that rapid polymerase chain reaction testing decreased antibiotic prescriptions as well as the likelihood of the patient returning for a second visit within two weeks.30
There are several types of point-of-care diagnostic tests for influenza. In otherwise heathy outpatients, more expensive rapid molecular assays may be preferred because of their higher sensitivity compared with other rapid testing methods.28 Because of the prolonged time to obtain results from viral culture or reference laboratory polymerase chain reaction testing, these tests are not practical for clinical decision-making in influenza management. Table 3 summarizes the accuracy and estimated positive and negative predictive values of point-of-care influenza tests in low- and high-prevalence populations.31
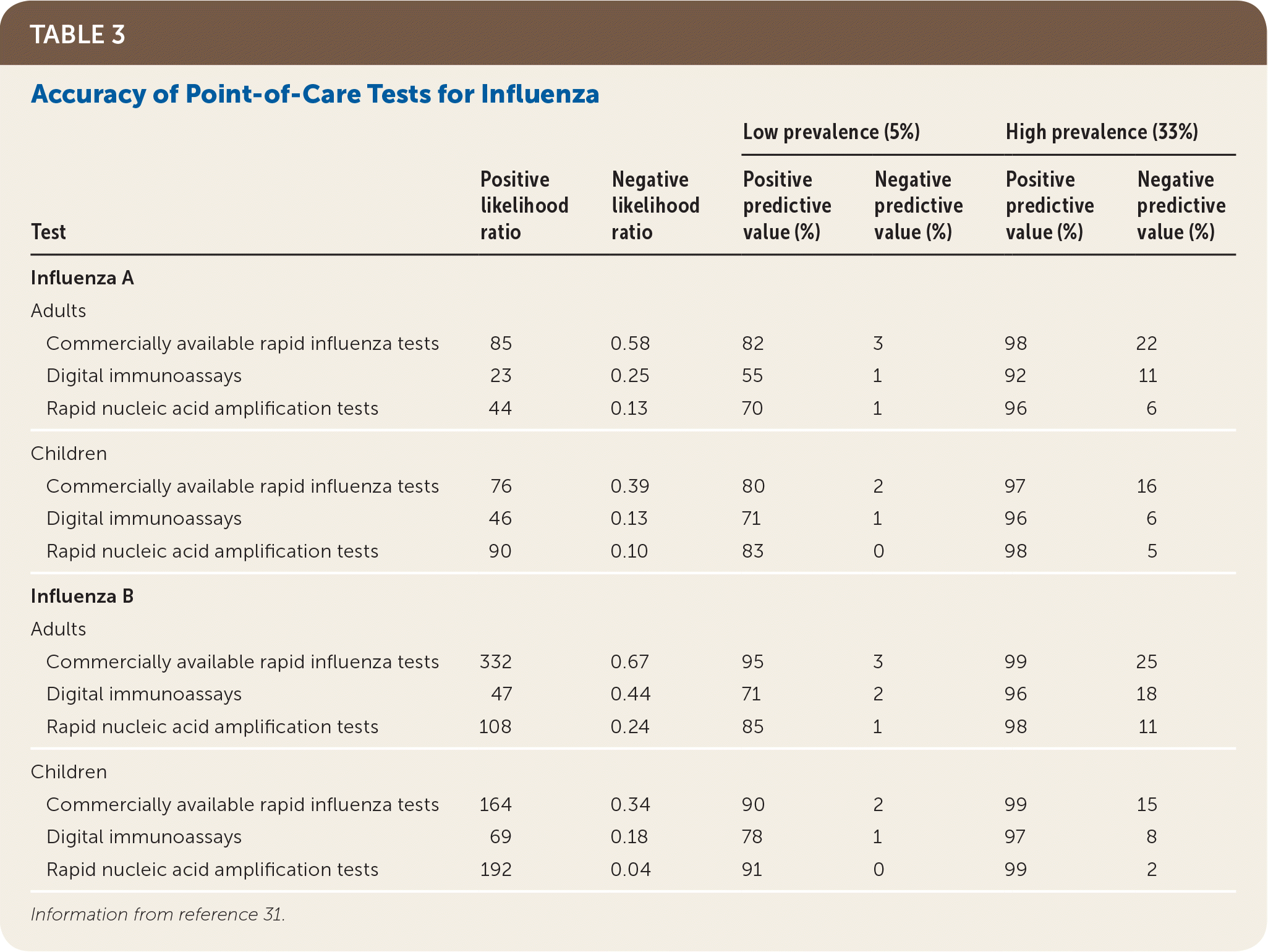
| Test | Positive likelihood ratio | Negative likelihood ratio | Low prevalence (5%) | High prevalence (33%) | ||
|---|---|---|---|---|---|---|
| Positive predictive value (%) | Negative predictive value (%) | Positive predictive value (%) | Negative predictive value (%) | |||
| Influenza A | ||||||
| Adults | ||||||
| Commercially available rapid influenza tests | 85 | 0.58 | 82 | 3 | 98 | 22 |
| Digital immunoassays | 23 | 0.25 | 55 | 1 | 92 | 11 |
| Rapid nucleic acid amplification tests | 44 | 0.13 | 70 | 1 | 96 | 6 |
| Children | ||||||
| Commercially available rapid influenza tests | 76 | 0.39 | 80 | 2 | 97 | 16 |
| Digital immunoassays | 46 | 0.13 | 71 | 1 | 96 | 6 |
| Rapid nucleic acid amplification tests | 90 | 0.10 | 83 | 0 | 98 | 5 |
| Influenza B | ||||||
| Adults | ||||||
| Commercially available rapid influenza tests | 332 | 0.67 | 95 | 3 | 99 | 25 |
| Digital immunoassays | 47 | 0.44 | 71 | 2 | 96 | 18 |
| Rapid nucleic acid amplification tests | 108 | 0.24 | 85 | 1 | 98 | 11 |
| Children | ||||||
| Commercially available rapid influenza tests | 164 | 0.34 | 90 | 2 | 99 | 15 |
| Digital immunoassays | 69 | 0.18 | 78 | 1 | 97 | 8 |
| Rapid nucleic acid amplification tests | 192 | 0.04 | 91 | 0 | 99 | 2 |
Treatment
Treatment with an anti-influenza drug is an option, with the decision to prescribe based on balancing potential benefits, harms, cost, and patient preferences. In otherwise healthy adults and children, the clinical benefit is greatest when treatment is initiated within 24 hours of symptom onset.28 Although anti-influenza drugs have been approved by the U.S. Food and Drug Administration for use within 48 hours of symptom onset, most clinical trials enrolled patients who had been symptomatic for no more than 36 hours. The primary benefit of treatment is a decrease in symptom duration by approximately 24 hours when treatment is initiated within 36 hours, and a reduction in disease severity.32,33 Among adults and children with influenza in the outpatient setting who are treated with an NA inhibitor, systematic reviews of published and unpublished randomized trials found no decrease in hospitalizations or death.29,33 However, in hospitalized adults and children, three observational studies found an association between the use of NA inhibitors and mortality benefit.34–36
The CDC and the IDSA recommend antiviral therapy for patients with severe or progressive illness, who are at high risk of influenza-associated complications, or who are hospitalized.18,28 Although early treatment is most beneficial, treatment should be initiated in these patients regardless of symptom duration.28 The IDSA also recommends that treatment be considered for household contacts of people at high risk of influenza-associated complications.28 Clinicians caring for high-risk patients can also be considered for treatment.28
Four antiviral drugs have been approved for the treatment of influenza (Table 4): the NA inhibitors oseltamivir (Tamiflu), zanamivir (Relenza), and peramivir (Rapivab), and the cap-dependent endonuclease inhibitor baloxavir (Xofluza).18,37 Any of these agents can be used in age-appropriate, otherwise healthy outpatients with uncomplicated influenza and no contraindications.18 Baloxavir is indicated for the treatment of uncomplicated influenza in patients 12 years and older.38 In a study comparing baloxavir with oseltamivir and placebo in 1,436 otherwise healthy people 12 to 65 years of age who had influenza, oseltamivir and baloxavir reduced symptom duration by approximately one day compared with placebo.38 Symptom duration was reduced by approximately 32 hours when oseltamivir or baloxavir was given within 24 hours of symptom onset, but only 13 hours if given 24 to 48 hours after onset.38 Given their cost, modest benefits, and adverse effects (primarily nausea and vomiting with oseltamivir), these drugs are not routinely recommended for otherwise healthy patients with influenza.39
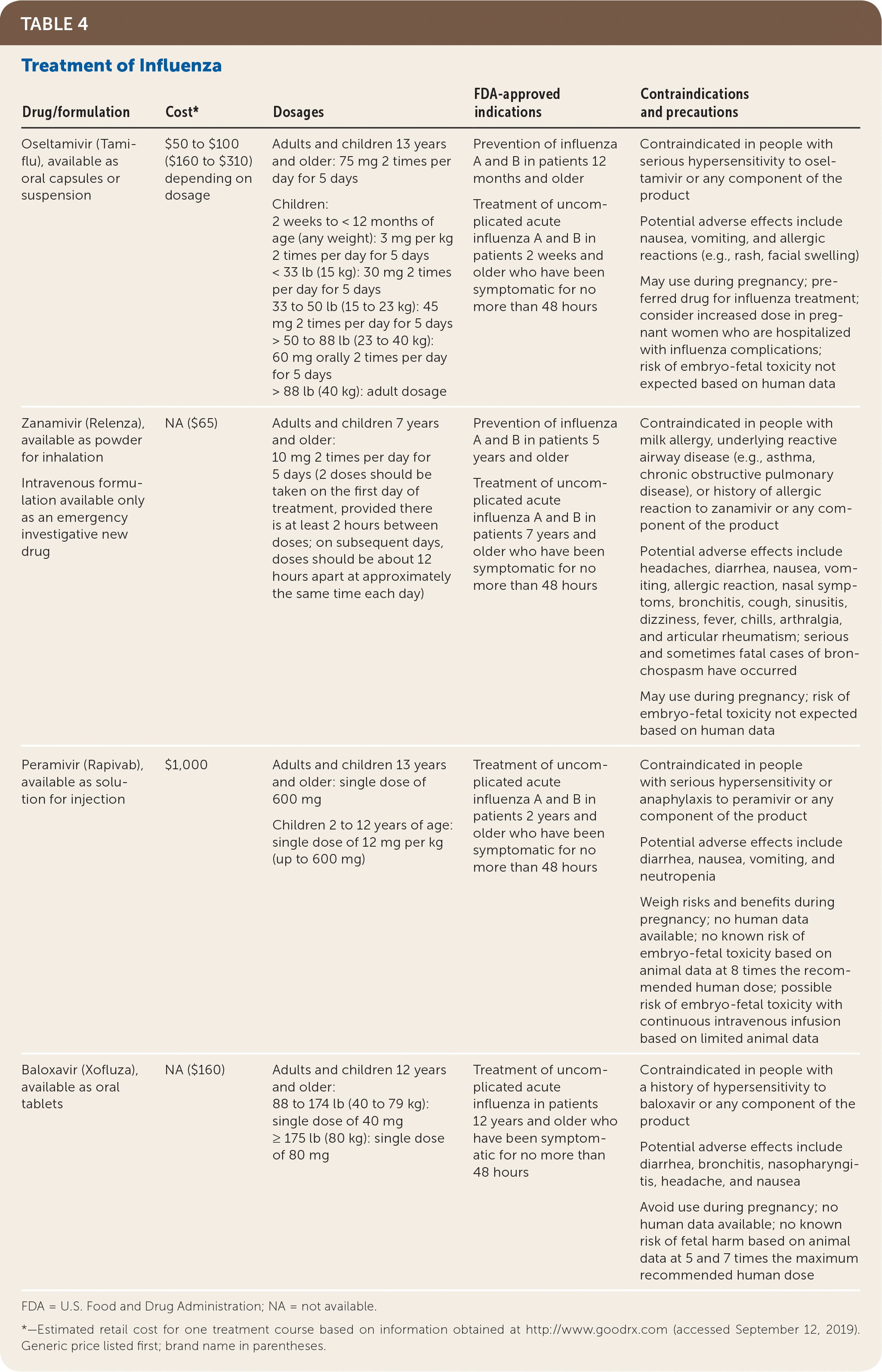
| Drug/formulation | Cost* | Dosages | FDA-approved indications | Contraindications and precautions |
|---|---|---|---|---|
| Oseltamivir (Tamiflu), available as oral capsules or suspension | $50 to $100 ($160 to $310) depending on dosage | Adults and children 13 years and older: 75 mg 2 times per day for 5 days Children: 2 weeks to < 12 months of age (any weight): 3 mg per kg 2 times per day for 5 days < 33 lb (15 kg): 30 mg 2 times per day for 5 days 33 to 50 lb (15 to 23 kg): 45 mg 2 times per day for 5 days > 50 to 88 lb (23 to 40 kg): 60 mg orally 2 times per day for 5 days > 88 lb (40 kg): adult dosage | Prevention of influenza A and B in patients 12 months and older Treatment of uncomplicated acute influenza A and B in patients 2 weeks and older who have been symptomatic for no more than 48 hours | Contraindicated in people with serious hypersensitivity to oseltamivir or any component of the product Potential adverse effects include nausea, vomiting, and allergic reactions (e.g., rash, facial swelling) May use during pregnancy; preferred drug for influenza treatment; consider increased dose in pregnant women who are hospitalized with influenza complications; risk of embryo-fetal toxicity not expected based on human data |
| Zanamivir (Relenza), available as powder for inhalation Intravenous formulation available only as an emergency investigative new drug | NA ($65) | Adults and children 7 years and older: 10 mg 2 times per day for 5 days (2 doses should be taken on the first day of treatment, provided there is at least 2 hours between doses; on subsequent days, doses should be about 12 hours apart at approximately the same time each day) | Prevention of influenza A and B in patients 5 years and older Treatment of uncomplicated acute influenza A and B in patients 7 years and older who have been symptomatic for no more than 48 hours | Contraindicated in people with milk allergy, underlying reactive airway disease (e.g., asthma, chronic obstructive pulmonary disease), or history of allergic reaction to zanamivir or any component of the product Potential adverse effects include headaches, diarrhea, nausea, vomiting, allergic reaction, nasal symptoms, bronchitis, cough, sinusitis, dizziness, fever, chills, arthralgia, and articular rheumatism; serious and sometimes fatal cases of bronchospasm have occurred May use during pregnancy; risk of embryo-fetal toxicity not expected based on human data |
| Peramivir (Rapivab), available as solution for injection | $1,000 | Adults and children 13 years and older: single dose of 600 mg Children 2 to 12 years of age: single dose of 12 mg per kg (up to 600 mg) | Treatment of uncomplicated acute influenza A and B in patients 2 years and older who have been symptomatic for no more than 48 hours | Contraindicated in people with serious hypersensitivity or anaphylaxis to peramivir or any component of the product Potential adverse effects include diarrhea, nausea, vomiting, and neutropenia Weigh risks and benefits during pregnancy; no human data available; no known risk of embryo-fetal toxicity based on animal data at 8 times the recommended human dose; possible risk of embryo-fetal toxicity with continuous intravenous infusion based on limited animal data |
| Baloxavir (Xofluza), available as oral tablets | NA ($160) | Adults and children 12 years and older: 88 to 174 lb (40 to 79 kg): single dose of 40 mg ≥ 175 lb (80 kg): single dose of 80 mg | Treatment of uncomplicated acute influenza in patients 12 years and older who have been symptomatic for no more than 48 hours | Contraindicated in people with a history of hypersensitivity to baloxavir or any component of the product Potential adverse effects include diarrhea, bronchitis, nasopharyngitis, headache, and nausea Avoid use during pregnancy; no human data available; no known risk of fetal harm based on animal data at 5 and 7 times the maximum recommended human dose |
Oseltamivir is the preferred treatment for patients with severe influenza. Intravenous peramivir is an option for these patients if there are contraindications to or concerns about reduced bioavailability of oral oseltamivir.18,28 Inhaled zanamivir is not recommended for patients with severe disease because it has not been well studied. It is contraindicated in patients requiring mechanical ventilation and in those with underlying lung disease because of the risk of bronchospasm.18,28 Adamantanes (amantadine and rimantadine [Flumadine]) are approved for influenza treatment but are not currently recommended. These medications are not active against influenza B, and most influenza A strains have shown adamantane resistance for the past 10 years.18
There is no demonstrated benefit to treating patients with more than one antiviral agent or using higher than recommended dosages.28 However, extended treatment courses may be indicated in critically ill patients.18 Supportive treatment and management of complications, including potential secondary bacterial pneumonia, are paramount. Corticosteroids are not recommended unless the patient has another approved indication for their use.18,28 Treatment resistance should be considered in patients who take antivirals and develop lower respiratory tract disease, although this is less likely than natural disease progression and more common in immunosuppressed patients.18
Pregnancy is an independent risk factor for complicated influenza. The risk of maternal death increases with each trimester and continues for four weeks postpartum.28 Oseltamivir has good safety data in pregnancy, and the CDC recommends it as first-line treatment for pregnant women.18 No change in dosing is necessary.
Chemoprophylaxis
Although chemoprophylaxis with NA inhibitors can prevent influenza infection, it is recommended only for specific populations18 (Table 4). Vaccination is the preferred method for prevention, and routine chemoprophylaxis within the community is not recommended.18 Clinicians can opt to closely monitor symptoms in exposed patients; early initiation of treatment is an alternative strategy.18,28 Indications to consider chemoprophylaxis are summarized in Table 5.18,28 However, most of these indications are based on poor-quality evidence and expert opinion. In a long-term care facility, an influenza outbreak is defined as two laboratory-confirmed cases within 72 hours in residents on the same unit.40 In the setting of outbreak in a nursing care facility or hospital, chemoprophylaxis is recommended for all asymptomatic patients and can be considered for certain staff (especially unvaccinated or recently vaccinated people).18

| Indications | Duration |
|---|---|
| Postexposure prophylaxis for people at high risk of complications of influenza should be initiated within 48 hours of initial exposure if unable to vaccinate or if vaccine effectiveness is likely to be low after close contact exposure to a person with influenza | 7 days after initial exposure |
| Postexposure prophylaxis in conjunction with vaccination for unvaccinated people in close contact with a person at high risk of influenza complications should be initiated within 48 hours of initial exposure to a person with influenza | At least 7 days after initial exposure or until vaccine effectiveness is likely achieved |
| Postexposure prophylaxis in unvaccinated people at high risk of influenza complications should be initiated within 48 hours of initial exposure in conjunction with vaccination after exposure to a person with influenza | 14 days or until vaccine effectiveness is likely achieved |
| Preexposure prophylaxis for people at high risk of developing complications of influenza if unable to vaccinate or vaccine likely to be ineffective when influenza is present (immune deficiency) | Duration of influenza activity |
| Preexposure prophylaxis for people at the highest risk of complications of influenza, such as hematopoietic stem cell transplant recipients within 12 months posttransplant or lung transplant recipients (people with severe immune deficiency) | Duration of influenza activity |
| Preexposure prophylaxis for unvaccinated people at high risk of complications of influenza in conjunction with vaccination when influenza activity is present in the community | 14 days or until vaccine effectiveness is likely achieved |
| Preexposure prophylaxis for unvaccinated people in close contact (including health care professionals) with people at high risk of influenza complications if unable to vaccinate, high-risk contacts cannot take chemoprophylaxis, and influenza activity is present in the community | Duration of influenza activity |
This article updates previous articles on this topic by Erlikh, et al.17 ; Montalto41 ; and Montalto, et al.42
Data Sources: We searched the websites of the Centers for Disease Control and Prevention and the Infectious Diseases Society of America, Essential Evidence Plus, the Cochrane database, PubMed, and UpToDate using the following search terms: influenza epidemiology, influenza neuraminidase, H1N1 pandemic, H1N1 pandemic mortality, H3N2 variant influenza, H5N1 influenza, influenza mortality, influenza complications, influenza complications American Indians, influenza treatment, and influenza universal vaccine. The search included meta-analyses, randomized controlled trials, clinical trials, and reviews. Search dates: December 1, 2018, to October 5, 2019.
The content presented is the opinion of the authors and does not reflect the views or policies of the U.S. Army, the Department of Defense, or the U.S. government. Mention of trade names, commercial products, or organizations does not imply an endorsement by the U.S. government.
