
Am Fam Physician. 2012;85(12):1194-1200
Author disclosure: No relevant financial affiliations to disclose.
Dutasteride/tamsulosin (Jalyn) is a combination 5-alpha reductase inhibitor and alpha1-adrenergic antagonist labeled for the treatment of symptomatic benign prostatic hyperplasia (BPH). The complementary mechanisms affect hormonal and smooth muscle pathways, respectively, to inhibit enlargement of the prostate and produce muscular relaxation to decrease symptoms.1
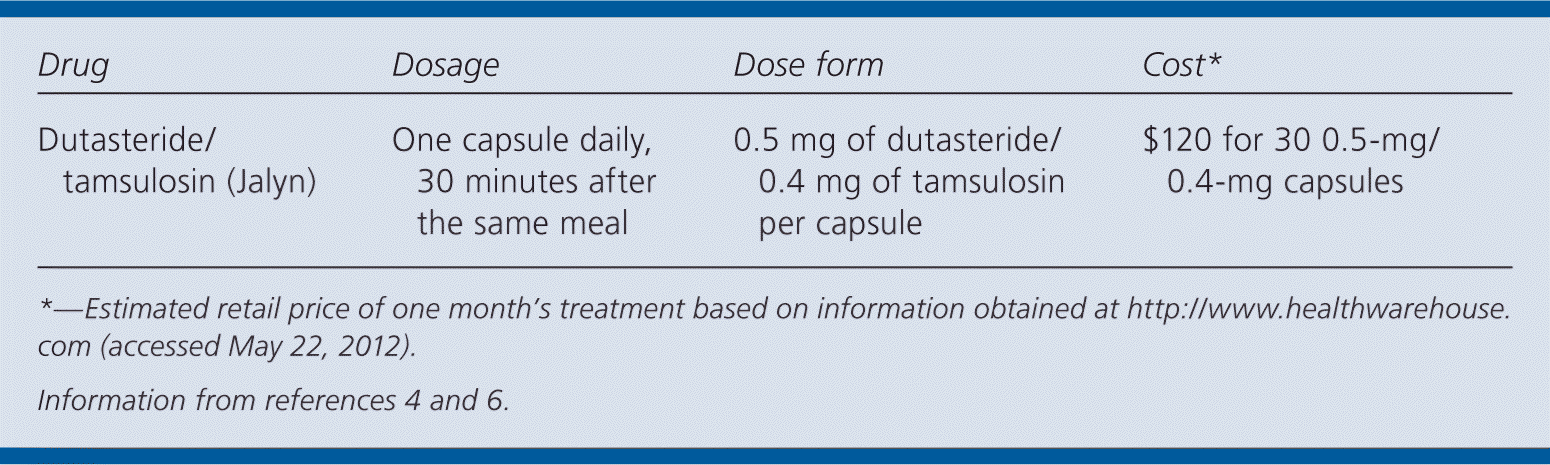
| Drug | Dosage | Dose form | Cost* |
|---|---|---|---|
| Dutasteride/tamsulosin (Jalyn) | One capsule daily, 30 minutes after the same meal | 0.5 mg of dutasteride/0.4 mg of tamsulosin per capsule | $120 for 30 0.5-mg/ 0.4-mg capsules |
SAFETY
Although dutasteride increases the incidence of high-grade prostate cancer, it has also been shown to reduce overall diagnoses of prostate cancer.2,3 The label specifically states that it does not prevent prostate cancer.1 Similar to dutasteride monotherapy, Jalyn decreases prostatespecific antigen (PSA) levels. The manufacturer of Jalyn recommends that PSA levels be evaluated when starting the drug, again three to six months after beginning treatment, and periodically thereafter. An increase in PSA levels could indicate prostate cancer, even when they are in the normal range.2
Medications with strong to moderate CYP3A4 or CYP2D6 inhibition properties (e.g., ketoconazole or paroxetine [Paxil], respectively) can increase blood levels of tamsulosin. Concomitant use of tamsulosin and alpha-adrenergic antagonists or phosphodiesterase type 5 inhibitors can increase the risk of orthostatic hypotension. Jalyn should not be used in patients with severe sulfa allergy because of rare reports of cross-reactivity with the tamsulosin component. Alternative treatment options include monotherapy with a 5-alpha reductase inhibitor, a phosphodiesterase type 5 inhibitor, cetrorelix (Cetrotide), and surgery. Jalyn is classified as a U.S. Food and Drug Administration pregnancy Category X drug because dutasteride may inhibit proper development of male genitalia.1 Because of this risk, and because the drug can be absorbed topically, women of childbearing age should not handle this medication.1
TOLERABILITY
The most common adverse effects of Jalyn are sexual in nature and include ejaculatory dysfunction, erectile dysfunction, decreased libido, and decreased amount of semen released during sex. These adverse effects will cause discontinuation of therapy in about 5 percent of men. Dizziness, breast swelling or tenderness, and runny nose are less common adverse effects, but also have been cited as reasons for discontinuation.1
EFFECTIVENESS
Jalyn was compared with dutasteride alone and tamsulosin alone in the CombAT (Combination of Avodart and Tamsulosin) study, which involved 4,844 carefully selected and monitored patients with moderate to severe BPH (International Prostate Symptom Score [IPSS] was 16.4 out of a possible 35).4 Compared with dutasteride or tamsulosin monotherapy, Jalyn decreased symptom scores by an average of 1.3 to 1.8 units more after two years of treatment, although the difference was not seen for at least nine months, and a difference of that magnitude is unlikely to be clinically significant.4 Patient-reported, disease-specific quality of life and treatment satisfaction also improved with Jalyn when compared with dutasteride or tamsulosin monotherapy after two years and four years of treatment, respectively.5
In the CombAT study, part of which included diagnosis using the IPSS, assessment of maximal urinary flow rate, and annual transrectal ultrasonography of the prostate, treatment with Jalyn was superior to tamsulosin monotherapy, but not to dutasteride monotherapy, for acute urinary retention (NNT = 22 for four years) and BPH-related surgery (NNT = 18 for four years).4 Jalyn was superior to either monotherapy at decreasing BPH clinical progression, which was defined as one of the following: symptom deterioration by IPSS of more than four points on two consecutive visits; BPH-related acute urinary retention; BPH-related urinary incontinence; BPH-related recurrent urinary tract infection or urosepsis; or BPH-related renal insufficiency (NNT = 11 for four years compared with tamsulosin monotherapy; NNT = 20 for four years compared with dutasteride monotherapy).4 In actual practice, the NNT will be higher (and the benefit lower), because these represent optimal circumstances for diagnosis, management, and surveillance.
PRICE
Jalyn costs about $120 for a 30-day supply of 30 capsules,6 which is less than the cost of purchasing dutasteride and tamsulosin separately. Generic tamsulosin is available at a much lower price (between $18 per 30 days6 and $160 per 90 days7). Dutasteride is $32 to $122 per 30 days.6,7 Insured patients may prefer Jalyn to avoid multiple copayments.
SIMPLICITY
The dosage of Jalyn is one capsule daily, taken 30 minutes after the same meal.
Bottom Line
Jalyn offers the convenience of taking one medication instead of two. Although monotherapy with dutasteride or tamsulosin is beneficial for many men, the combination drug is slightly better than either component alone at decreasing symptom scores; however, these benefits are of questionable clinical significance. In carefully diagnosed, selected, and monitored patients who do not respond to monotherapy, there may be some clinical benefit.
