A more recent article on peptic ulcer disease and H. pylori infection is available.
Am Fam Physician. 2015;91(4):236-242
Patient information: See related handout on H. pylori and stomach ulcers, written by the authors of this article.
Author disclosure: No relevant financial affiliations.
The most common causes of peptic ulcer disease (PUD) are Helicobacter pylori infection and use of nonsteroidal anti-inflammatory drugs (NSAIDs). The test-and-treat strategy for detecting H. pylori is appropriate in situations where the risk of gastric cancer is low based on age younger than 55 years and the absence of alarm symptoms. Most other patients should undergo upper endoscopy to rule out malignancy and other serious causes of dyspepsia. Urea breath tests and stool antigen tests are most accurate for identifying H. pylori infection and can be used to confirm cure; serologic tests are a convenient but less accurate alternative and cannot be used to confirm cure. Treatment choices include standard triple therapy, sequential therapy, quadruple therapy, and levofloxacin-based triple therapy. Standard triple therapy is only recommended when resistance to clarithromycin is low. Chronic use of NSAIDs in patients with H. pylori infection increases the risk of PUD. Recommended therapies for preventing PUD in these patients include misoprostol and proton pump inhibitors. Complications of PUD include bleeding, perforation, gastric outlet obstruction, and gastric cancer. Older persons are at higher risk of PUD because of high-risk medication use, including antiplatelet drugs, warfarin, selective serotonin reuptake inhibitors, and bisphosphonates.
Dyspepsia is characterized by epigastric pain, discomfort, or a burning sensation.1 An important cause of dyspepsia is peptic ulcer disease (PUD), which includes gastric and duodenal ulcers. Although PUD is most commonly caused by Helicobacter pylori infection or use of nonsteroidal anti-inflammatory drugs (NSAIDs), other diagnoses should be considered (Table 1).
| Clinical recommendation | Evidence rating | References |
|---|---|---|
| Use the test-and-treat strategy for patients with dyspepsia who are younger than 55 years and have no alarm symptoms for gastric cancer. Use endoscopy for all other patients. | A | 1–4 |
| Confirm eradication of Helicobacter pylori after therapy in patients with H. pylori–associated ulcer, continued dyspeptic symptoms, mucosa-associated lymphoid tissue lymphoma, and resection of gastric cancer. | C | 2 |
| Non–bismuth-based quadruple therapy (10 days of a proton pump inhibitor, amoxicillin 1 g, clarithromycin 500 mg [Biaxin], and metronidazole 500 mg [Flagyl] or tinidazole 500 mg [Tindamax] twice daily) has the highest success rate in eradicating H. pylori, although other regimens may also be used. | A | 12, 13 |
| For patients at low risk of gastrointestinal complications, nonsteroidal anti-inflammatory drugs may be used, whereas cotherapy with a proton pump inhibitor or misoprostol (Cytotec) is recommended for patients with moderate risk of ulcer, and they should be avoided in those with a high risk of ulcer. | C | 22, 24 |
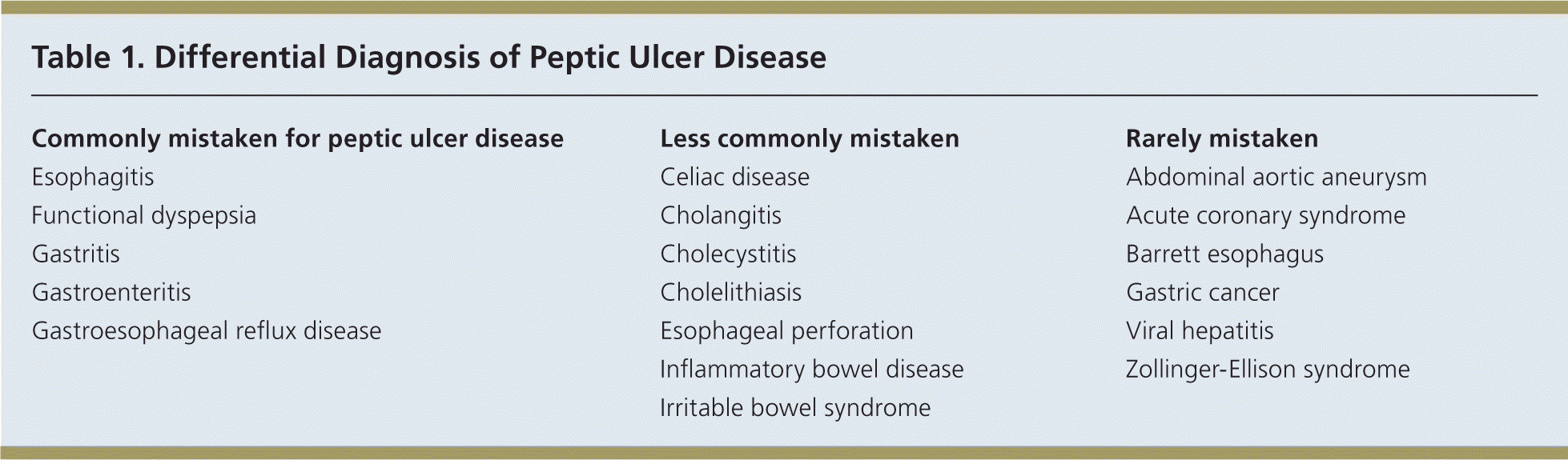
| Commonly mistaken for peptic ulcer disease |
| Esophagitis |
| Functional dyspepsia |
| Gastritis |
| Gastroenteritis |
| Gastroesophageal reflux disease |
| Less commonly mistaken |
| Celiac disease |
| Cholangitis |
| Cholecystitis |
| Cholelithiasis |
| Esophageal perforation |
| Inflammatory bowel disease |
| Irritable bowel syndrome |
| Rarely mistaken |
| Abdominal aortic aneurysm |
| Acute coronary syndrome |
| Barrett esophagus |
| Gastric cancer |
| Viral hepatitis |
| Zollinger-Ellison syndrome |
Pathophysiology of H. pylori
H. pylori, a gram-negative, helical, rod-shaped bacterium, colonizes the gastric mucosa of approximately one-half of the world population2 and an estimated 30% to 40% of the U.S. population.3 H. pylori is present in 95% of patients with duodenal ulcers and in 70% of those with gastric ulcers.4 It is typically transmitted via the fecal-oral route during early childhood and persists for decades. The bacterium is a known cause of gastric and duodenal ulcers5 and is a risk factor for mucosa-associated lymphoid tissue (MALT) lymphoma and gastric adenocarcinoma.6,7
Diagnosis
The history and physical examination are important to identify patients at risk of ulcer, perforation, bleeding, or malignancy. However, a systematic review of models using risk factors, history, and symptoms found that they did not reliably distinguish between functional dyspepsia and organic disease.8 Therefore, the test-and-treat strategy for H. pylori is recommended for patients with dyspepsia who have no alarm symptoms.1
The American College of Gastroenterology (ACG) recommends testing for H. pylori infection in patients with active PUD or history of PUD, dyspepsia symptoms, or gastric MALT lymphoma.3 The rationale for testing patients with a history of PUD who are currently asymptomatic is that detecting and treating H. pylori infection can reduce the risk of recurrence. The test-and-treat strategy for detecting H. pylori is appropriate in patients with dyspepsia and low risk of gastric cancer (age younger than 55 years and no alarm symptoms such as unexplained weight loss, progressive dysphagia, odynophagia, recurrent vomiting, family history of gastrointestinal cancer, overt gastrointestinal bleeding, abdominal mass, iron deficiency anemia, or jaundice).1–4 Endoscopy is recommended for patients who are 55 years or older, or who have alarm symptoms. The accuracy of diagnostic tests for H. pylori infection is summarized in Table 2.9,10
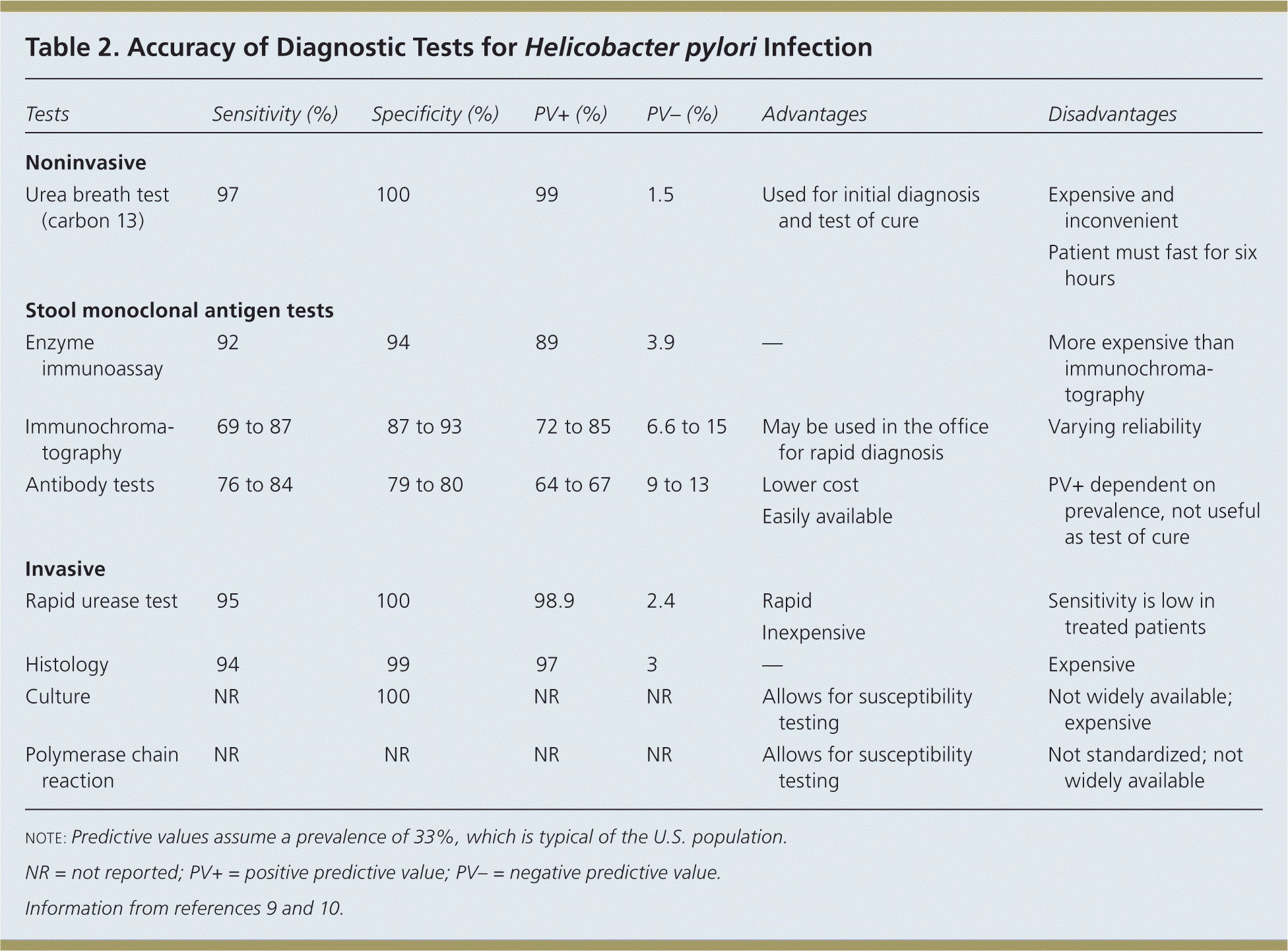
| Tests | Sensitivity (%) | Specificity (%) | PV+ (%) | PV– (%) | Advantages | Disadvantages | |
|---|---|---|---|---|---|---|---|
| Noninvasive | |||||||
| Urea breath test (carbon 13) | 97 | 100 | 99 | 1.5 | Used for initial diagnosis and test of cure |
| |
| Stool monoclonal antigen tests | |||||||
| Enzyme immunoassay | 92 | 94 | 89 | 3.9 | — | More expensive than immunochromatography | |
| Immunochromatography | 69 to 87 | 87 to 93 | 72 to 85 | 6.6 to 15 | May be used in the office for rapid diagnosis | Varying reliability | |
| Antibody tests | 76 to 84 | 79 to 80 | 64 to 67 | 9 to 13 | Lower cost | PV+ dependent on prevalence, not useful as test of cure | |
| Easily available | |||||||
| Invasive | |||||||
| Rapid urease test | 95 | 100 | 98.9 | 2.4 | Rapid | Sensitivity is low in treated patients | |
| Inexpensive | |||||||
| Histology | 94 | 99 | 97 | 3 | — | Expensive | |
| Culture | NR | 100 | NR | NR | Allows for susceptibility testing | Not widely available; expensive | |
| Polymerase chain reaction | NR | NR | NR | NR | Allows for susceptibility testing | Not standardized; not widely available | |
UREA BREATH TESTS
Urea breath tests require the ingestion of urea labeled with the nonradioactive isotope carbon 13 or carbon 14. Specificity and sensitivity approach 100%. Urea breath testing is one option for test of cure and should be performed four to six weeks after completion of eradication therapy. Proton pump inhibitors (PPIs) must be stopped for at least two weeks before the test, and accuracy is lower in patients who have had distal gastrectomy. Cost and inconvenience are disadvantages of this test.8
STOOL MONOCLONAL ANTIGEN TESTS
Stool antigen tests using monoclonal antibodies are as accurate as urea breath tests if a validated laboratory-based monoclonal test is used.1,11 They are cheaper and require less equipment than urea breath tests. Like urea breath tests, stool antigen tests detect only active infection and can be used as a test of cure. PPIs should be stopped for two weeks before testing, but stool antigen tests are not as affected by PPI use as are urea breath tests.
SEROLOGIC TESTS
Serologic antibody testing detects immunoglobulin G specific to H. pylori in serum and cannot distinguish between an active infection and a past infection. Serologic tests may be most useful in mass population surveys and in patients who cannot stop taking PPIs (e.g., those with gastrointestinal bleeding or continuous NSAID use) because the tests are not affected by PPI or antibiotic use.1,2
ENDOSCOPY WITH BIOPSY
Endoscopy with biopsy is recommended to rule out cancer and other serious causes in patients 55 years or older, or with one or more alarm symptoms. In patients who have not been taking a PPI within one to two weeks of endoscopy, or bismuth or an antibiotic within four weeks, the rapid urease test performed on the biopsy specimen provides an accurate, inexpensive means of diagnosing H. pylori infection.2 Patients who have been on these medications will require histology, with or without rapid urease testing. Culture and polymerase chain reaction allow for susceptibility testing but are not readily available for clinical use in the United States.
Treatment
Eradication of H. pylori is recommended in all patients with PUD.1 First-line therapy should have an eradication rate of more than 80%.4 Because pretreatment susceptibility is rarely known to the primary care physician, therapy must be chosen empirically based on regional bacterial resistance patterns, local recommendations, and drug availability. Table 3 includes treatment options; standard triple therapy is a reasonable initial therapy where clarithromycin resistance is low.2–4,12–16
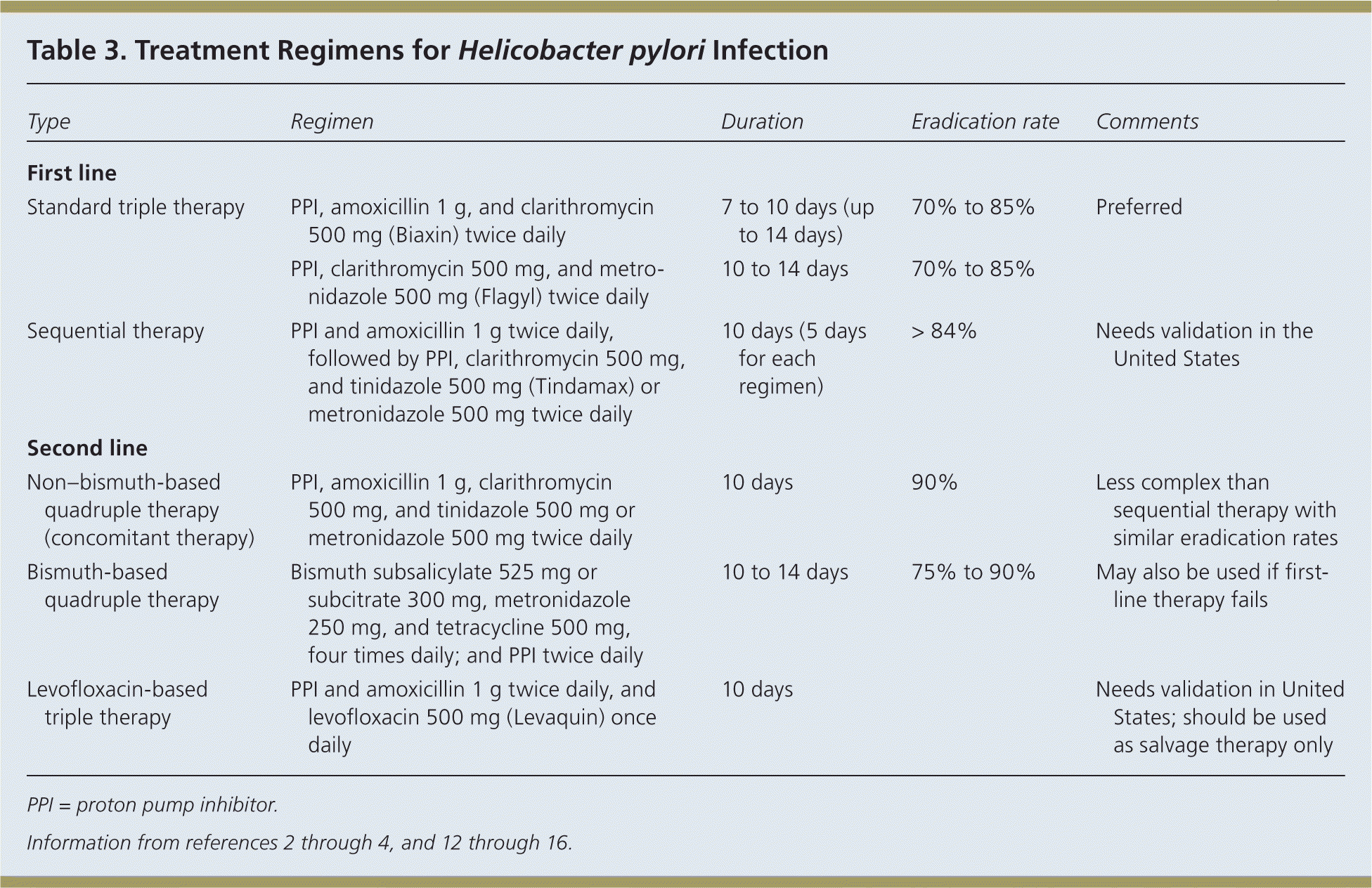
| Type | Regimen | Duration | Eradication rate | Comments |
|---|---|---|---|---|
| First line | ||||
| Standard triple therapy | PPI, amoxicillin 1 g, and clarithromycin 500 mg (Biaxin) twice daily | 7 to 10 days (up to 14 days) | 70% to 85% | Preferred |
| PPI, clarithromycin 500 mg, and metronidazole 500 mg (Flagyl) twice daily | 10 to 14 days | 70% to 85% | ||
| Sequential therapy | PPI and amoxicillin 1 g twice daily, followed by PPI, clarithromycin 500 mg, and tinidazole 500 mg (Tindamax) or metronidazole 500 mg twice daily | 10 days (5 days for each regimen) | > 84% | Needs validation in the United States |
| Second line | ||||
| Non–bismuth-based quadruple therapy (concomitant therapy) | PPI, amoxicillin 1 g, clarithromycin 500 mg, and tinidazole 500 mg or metronidazole 500 mg twice daily | 10 days | 90% | Less complex than sequential therapy with similar eradication rates |
| Bismuth-based quadruple therapy | Bismuth subsalicylate 525 mg or subcitrate 300 mg, metronidazole 250 mg, and tetracycline 500 mg, four times daily; and PPI twice daily | 10 to 14 days | 75% to 90% | May also be used if first-line therapy fails |
| Levofloxacin-based triple therapy | PPI and amoxicillin 1 g twice daily, and levofloxacin 500 mg (Levaquin) once daily | 10 days | Needs validation in United States; should be used as salvage therapy only | |
Eradication heals most duodenal ulcers and greatly diminishes the risk of recurrent bleeding.3 A systematic review found that treatment of H. pylori infection is more effective than antisecretory noneradicating therapy (with or without long-term maintenance antisecretory therapy) in preventing recurrent bleeding from peptic ulcer.17 Current data suggest that increasing the duration of therapy to 14 days significantly increases the eradication rate.18
TEST OF CURE
Test of cure for all patients after therapy is neither cost-effective nor practical. Indications for eradication testing with the urea breath test or stool antigen test include H. pylori–associated ulcer, continued dyspeptic symptoms, H. pylori–associated MALT lymphoma, and resection for gastric cancer.2 When indicated, eradication testing should be performed at least four weeks after completion of therapy.2
STANDARD TRIPLE THERAPY
A seven- to 10-day triple drug regimen consisting of a PPI, amoxicillin 1 g, and clarithromycin 500 mg (Biaxin) twice daily has long been the first-line therapy to eradicate H. pylori. However, increasing resistance to clarithromycin is associated with declining eradication rates, now well below 80%.19 Therefore, this regimen is not recommended where the prevalence of clarithromycin-resistant strains of H. pylori exceeds 15% to 20%.1 An alternative triple drug regimen substitutes metronidazole 500 mg twice daily for amoxicillin. Adding probiotics to triple therapy, specifically Saccharomyces boulardii and Lactobacillus, has been shown to increase eradication rates (absolute increase of 9% and 5%, respectively) and decrease adverse effects of treatment, particularly diarrhea (absolute decrease of 14% and 7%, respectively).20,21
SEQUENTIAL THERAPY
Sequential therapy consists of a five-day course of a PPI and amoxicillin 1 g taken twice daily, followed by a five-day course of a PPI, clarithromycin 500 mg, and metronidazole 500 mg (Flagyl) or tinidazole 500 mg (Tindamax) taken twice daily. The overall eradication rate is 84%, with an eradication rate of 73% for clarithromycin-resistant strains. A recent meta-analysis of available global data revealed that sequential therapy is superior to seven-day triple therapy, but it is not superior to 14-day triple therapy, bismuth-based quadruple therapy, or non–bismuth-based quadruple therapy.12
Compliance and tolerance rates of sequential therapy are similar to those of triple therapy but cost is lower, especially when the cost of failure of first-line therapy is considered. However, most studies were performed in Italy, and the ACG guideline states that sequential therapy requires validation in the United States.3
NON–BISMUTH-BASED QUADRUPLE THERAPY (CONCOMITANT THERAPY)
This approach involves the addition of metronidazole 500 mg or tinidazole 500 mg twice daily to the standard triple regimen. It is less complex than sequential therapy with similar eradication rates.13,14 Additionally, non–bismuth-based quadruple therapy may be more effective than sequential therapy in patients with dual antibiotic resistance to clarithromycin and metronidazole.15 It has the highest eradication rate, about 90%, even in areas with high clarithromycin and metronidazole resistance,22,23 but would presumably cost more than sequential therapy because clarithromycin is taken for 10 days.
BISMUTH-BASED QUADRUPLE THERAPY
This is the traditional quadruple regimen and includes a bismuth salt (subsalicylate 525 mg or subcitrate potassium 420 mg), metronidazole 250 mg, and tetracycline 375 to 500 mg, all taken four times daily, in addition to a PPI taken twice per day.2 Bismuth-based quadruple therapy is often employed as salvage therapy if first-line treatment fails, but it may be used as first-line therapy in areas of high resistance or when cost is an important consideration. A three-in-one combination capsule containing bismuth subcitrate potassium, metronidazole, and tetracycline has been developed to help reduce the pill burden, but patients still have to take three capsules four times per day in addition to a PPI. The regimen is usually given for 10 to 14 days.
LEVOFLOXACIN-BASED TRIPLE THERAPY
NSAIDs and PUD
PREVENTION
Risk factors for gastrointestinal toxicity from NSAID use include older age; chronic use of high-dose NSAIDs; use of aspirin, anticoagulants, or corticosteroids; and a history of ulcer.22 Therapies aimed at protecting the mucosa include the prostaglandin analogue misoprostol (Cytotec), histamine H2 receptor antagonists, a cyclooxygenase-2 (COX-2) inhibitor instead of a standard NSAID, and PPIs. A Cochrane review on the effectiveness of these therapies compared with placebo suggests that high-risk patients should take a COX-2 inhibitor with a PPI for the greatest gastrointestinal safety.23
Concerns have been raised about increased cardiovascular risk with the use of COX-2 inhibitors. The ACG22 and the Canadian Association of Gastroenterology 24 have each developed evidence-based guidelines for the prevention of NSAID-related ulcers in patients at risk of cardiovascular disease, including those with previous cardiovascular events. The recommendations are summarized in Table 4.22,24 NSAIDs are appropriate for patients with low risk of gastrointestinal complications, whereas cotherapy with a PPI or misoprostol is preferred for patients with gastrointestinal risk factors.22,24 Patients at low cardiovascular risk may take traditional NSAIDs or a COX-2 inhibitor; however, the Canadian Association of Gastroenterology suggests that the use of naproxen may be appropriate for patients at high cardiovascular risk.22,24
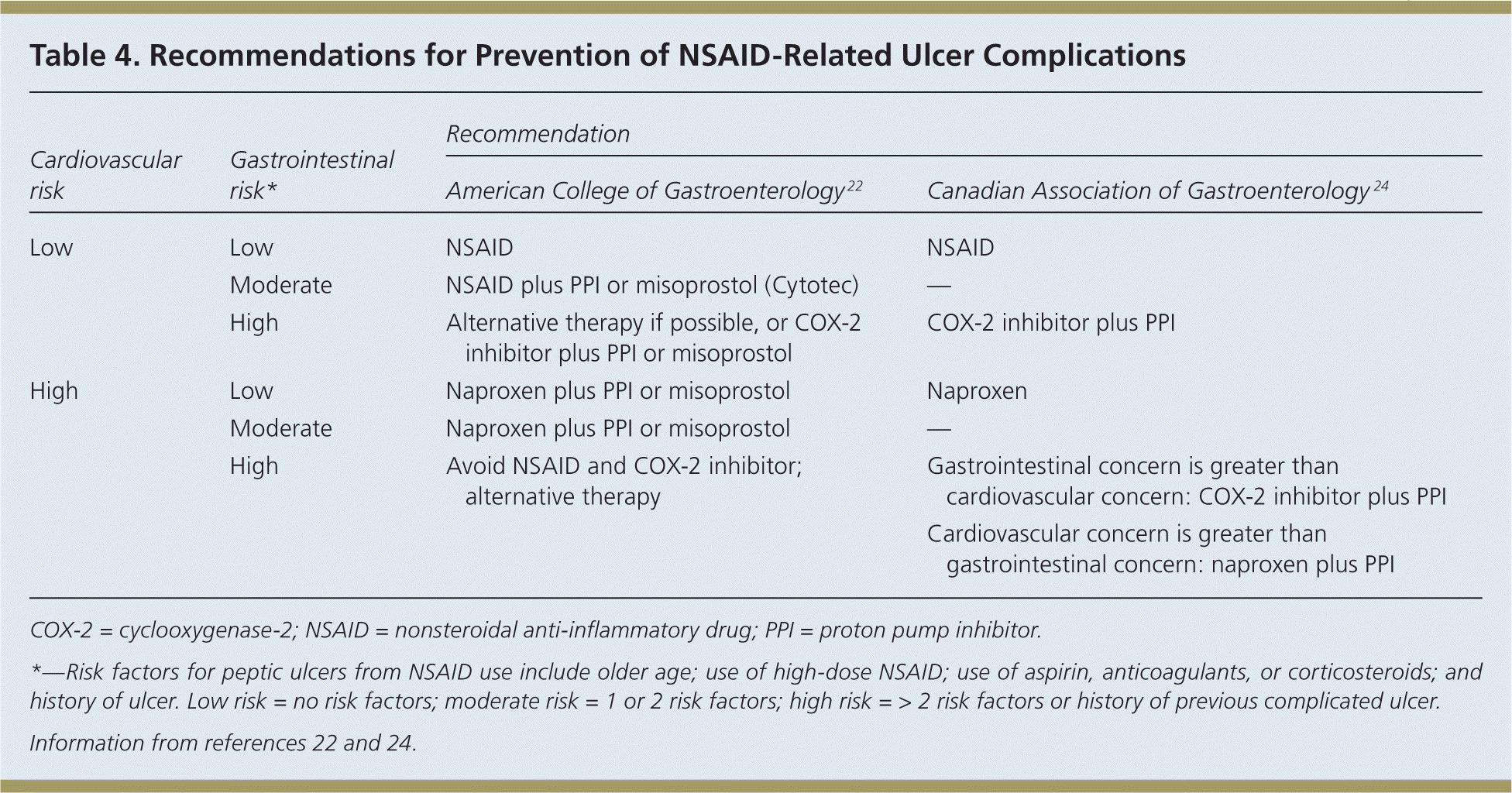
| Cardiovascular risk | Gastrointestinal risk* | Recommendation | |
|---|---|---|---|
| American College of Gastroenterology 22 | Canadian Association of Gastroenterology 24 | ||
| Low | Low | NSAID | NSAID |
| Moderate | NSAID plus PPI or misoprostol (Cytotec) | — | |
| High | Alternative therapy if possible, or COX-2 inhibitor plus PPI or misoprostol | COX-2 inhibitor plus PPI | |
| High | Low | Naproxen plus PPI or misoprostol | Naproxen |
| Moderate | Naproxen plus PPI or misoprostol | — | |
| High | Avoid NSAID and COX-2 inhibitor; alternative therapy | Gastrointestinal concern is greater than cardiovascular concern: COX-2 inhibitor plus PPI | |
| Cardiovascular concern is greater than gastrointestinal concern: naproxen plus PPI | |||
NSAIDS AND H. PYLORI
Peptic ulcers are more common in patients taking NSAIDs who are H. pylori positive compared with those who are negative (pooled odds ratio [OR] = 1.81; 95% confidence interval [CI], 1.40 to 2.36). Bleeding is also more likely to occur in patients taking NSAIDs who are H. pylori positive (pooled OR = 5.21; 95% CI, 3.48 to 7.78).5 Eradicating H. pylori in NSAID users reduces the likelihood of peptic ulcer by about one-half (OR = 0.43; 95% CI, 0.2 to 0.9).25 However, a meta-analysis found that the use of a maintenance PPI was more effective than H. pylori eradication therapy for preventing NSAID-related ulcers (OR = 7.4; 95% CI, 1.3 to 44).25 The ACG guideline recommends that patients who will be on long-term NSAID therapy be tested for H. pylori infection, and eradication therapy should be given if positive.22
Special Circumstances in PUD
OLDER PERSONS
Older persons are at a higher risk of PUD, in part because of high-risk medication use, including antiplatelet drugs, warfarin (Coumadin), selective serotonin reuptake inhibitors, and bisphosphonates.26 Compared with younger patients, older patients have less abdominal pain when they have an ulcer.27 Physicians should identify other risk factors for ulcers when older patients are taking NSAIDs, including previous ulcer, use of anti-platelet or anticoagulant medications, smoking, severe comorbidity or frailty, and alcohol abuse.26 Treatment options include discontinuing or reducing the dose of NSAIDs, choosing a less damaging NSAID or changing to a COX-2 inhibitor, or starting a PPI or misoprostol. After eradication of H. pylori, older patients taking an NSAID may still need a maintenance PPI.26 However, long-term PPI use is associated with an increased risk of Clostridium difficile–associated diarrhea, community-acquired pneumonia, interstitial nephritis, osteoporosis, and some vitamin and mineral malabsorptions.28
CHILDREN
Although gastrointestinal symptoms are common in children, PUD is rare (24.8 per 100,000 children annually).29 Recurrent abdominal pain is not associated with H. pylori infection, and there is conflicting evidence regarding the association between epigastric pain and H. pylori infection.30 One study found that nausea, vomiting, and diarrhea were associated with H. pylori, but that abdominal pain and heartburn were not.31 An evidence-based clinical guideline developed by an international panel makes recommendations for H. pylori infection in children and adolescents. The best supported recommendations are presented in Table 5.32

| Physicians should wait at least 2 weeks after the patient stops taking a proton pump inhibitor or 4 weeks after the patient stops taking an antibiotic to perform biopsy-based or noninvasive testing (e.g., urea breath test, stool test) for H. pylori. |
| Diagnostic testing for H. pylori is not recommended in children with functional abdominal pain. |
| In patients with peptic ulcer disease and H. pylori infection, the organism should be eradicated. |
| Tests based on the detection of antibodies (immunoglobulin G or A) against H. pylori are not reliable in clinical setting. |
| The urea breath test (carbon 13) is a reliable noninvasive test to determine whether H. pylori has been eradicated. |
Complications
The complications of PUD from any etiology include bleeding, perforation, and gastric outlet obstruction.33 Hemorrhage is the most common complication, with up to 15% of patients experiencing some degree of bleeding. In one study, the incidence of peptic ulcer hospitalizations was 5.65 per 1,000 person-years in patients taking NSAIDs without gastrointestinal protective therapy.33 Endoscopy is considered the standard of care for patients with gastrointestinal bleed and may allow for treatment of the ulcer at the same time.29
The incidence of perforation from PUD in the general population (not taking NSAIDs) is about one per 10,000 person-years.34 Perforation leads to peritonitis from the release of contents into the abdominal cavity.33 Patients with perforation will have intense abdominal pain, and because the ulcer may perforate to a nearby organ such as the liver or pancreas, amylase, lipase, and hepatic transaminases may be affected. If the perforation is treated quickly, the mortality rate is 6% to 14%, with poorer outcomes in patients with advanced age or major illness.33
The duodenum can become narrowed from continued inflammation and scarring from ulcers, which may lead to gastric outlet obstruction.33 Patients usually present with severe vomiting and hematemesis. Gastric outlet obstruction is rare, and physicians should consider an underlying malignancy in these patients.35
Gastric cancer is the second leading cause of cancer-related mortality.36 H. pylori has an epidemiologic role in the multifactorial process of gastric carcinogenesis.37 It has virulence factors that directly influence cell transformation in those who have chronic H. pylori infection. It also causes chronic inflammation with an exaggerated immune response, which results in carcinogenesis. Although 50% of the world population is infected with H. pylori, less than 2% ever develop gastric cancer.35
Data Sources: Essential Evidence Plus was searched using the key words duodenal ulcer, Helicobacter, Helicobacter infections, peptic ulcer, and stomach ulcer. This yielded InfoPOEMs, Cochrane reviews, and practice guidelines. The Trip database was also searched using the key words Helicobacter pylori and nonsteroidal anti-inflammatory drugs. Search date: November 27, 2013.