Am Fam Physician. 2015;91(4):250-256
A more recent article on lung cancer is available.
Related editorials: Should Family Physicians Routinely Screen for Lung Cancer in High-Risk Populations? Yes: CT-Based Screening Is Complex but Worthwhile and No: The USPSTF's Recommendation for Lung Cancer Screening Is Overreaching
Patient information: See related handout on lung cancer, written by the authors of this article.
Author disclosure: No relevant financial affiliations.
Lung cancer is classified histologically into small cell and non–small cell lung cancers. The most common symptoms of lung cancer are cough, dyspnea, hemoptysis, and systemic symptoms such as weight loss and anorexia. High-risk patients who present with symptoms should undergo chest radiography. If a likely alternative diagnosis is not identified, computed tomography and possibly positron emission tomography should be performed. If suspicion for lung cancer is high, a diagnostic evaluation is warranted. The diagnostic evaluation has three simultaneous steps (tissue diagnosis, staging, and functional evaluation), all of which affect treatment planning and determination of prognosis. The least invasive method possible should be used. The diagnostic evaluation and treatment of a patient with lung cancer require a team of specialists, including a pulmonologist, medical oncologist, radiation oncologist, pathologist, radiologist, and thoracic surgeon. Non–small cell lung cancer specimens are tested for various mutations, which, if present, can be treated with new targeted molecular therapies. The family physician should remain involved in the patient's care to ensure that the values and wishes of the patient and family are considered and, if necessary, to coordinate end-of-life care. Early palliative care improves quality of life and may prolong survival. Family physicians should concentrate on early recognition of lung cancer, as well as prevention by encouraging tobacco cessation at every visit. The U.S. Preventive Services Task Force recommends lung cancer screening using low-dose computed tomography in high-risk patients. However, the American Academy of Family Physicians concludes that the evidence is insufficient to recommend for or against screening. Whether to screen high-risk patients should be a shared decision between the physician and patient.
In 2010, approximately 200,000 persons in the United States were diagnosed with lung cancer, and nearly 160,000 persons died of the disease.1,2 The average age at diagnosis is 68 to 70 years. Incidence and death rates vary widely among states and regions of the United States, commensurate with geographic disparities in tobacco use. Utah has the lowest incidence (28 per 100,000) and Kentucky has the highest (101 per 100,000).4 The overall incidence of lung cancer declined by 2% between 2005 and 2009.5
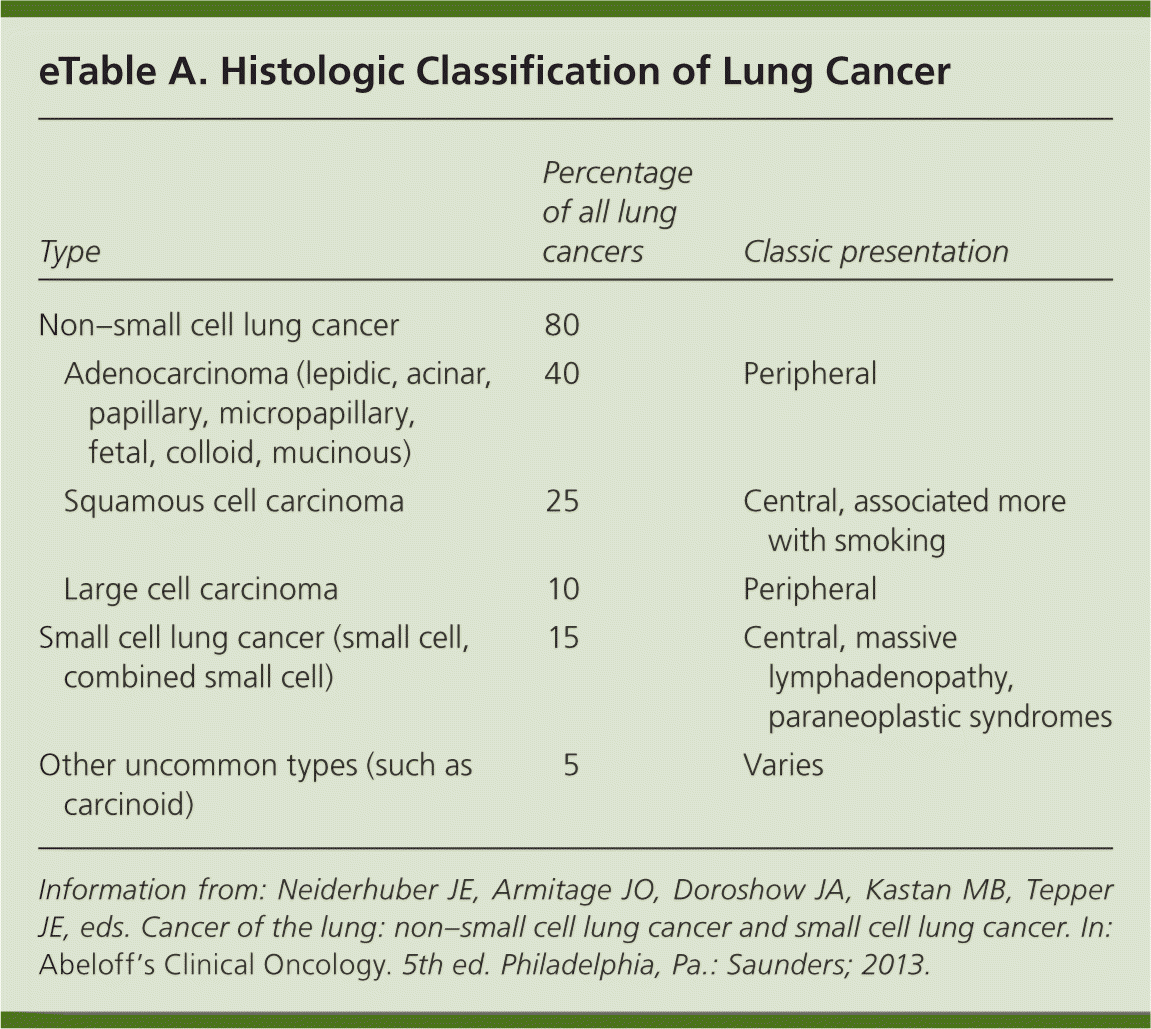
| Clinical recommendation | Evidence rating | References |
|---|---|---|
| Chest radiography should be performed in patients with signs and symptoms consistent with lung cancer, and contrast-enhanced computed tomography should be performed if a likely alternative diagnosis is not identified on the chest radiograph. | C | 17, 19, 22 |
| The diagnosis of suspected lung cancer should be confirmed using the least invasive method possible. | C | 22 |
| Endobronchial ultrasound and electromagnetic navigation can increase the diagnostic yield of bronchoscopy for mediastinal or peripheral lesions. | C | 23, 24 |
| Medically fit patients with infiltrative stage III non–small cell lung cancer should be offered chemotherapy and radiation therapy. | A | 31 |
| Patients with stage III non–small cell lung cancer should receive chemotherapy and radiation therapy. | A | 32 |
| Early limited stage small cell lung cancer is treated with chemotherapy and radiation therapy, and possibly surgery in the earliest stages. | C | 34 |
| Early palliative care results in improved quality of life and a decreased incidence of depression in patients with newly diagnosed non–small cell lung cancer. | B | 33 |
| Consider screening high-risk patients for lung cancer annually with low-dose computed tomography (number needed to screen of 312 to prevent one death). | A | 35, 36 |
| Recommendation | Sponsoring organization |
|---|---|
| Do not perform computed tomography screening for lung cancer among patients at low risk of lung cancer. | American College of Chest Physicians/American Thoracic Society |
Risk Factors
Tobacco use causes 80% to 90% of all lung cancers.6,7 Secondhand tobacco smoke exposure is also a significant risk factor, with younger age at exposure associated with higher risk of lung cancer.8 Risk factors (Table 16–14 ) are typically dose- and duration-dependent, and many carcinogens act synergistically when combined with tobacco smoke.9 For example, arsenic in drinking water has been associated with lung cancer when combined with exposure to tobacco smoke.10,11 Radon, a naturally occurring radioactive gas found in some homes, is estimated to cause 21,000 cases of lung cancer per year.12 Any home can have elevated radon levels, but the highest levels are found in the Northern and Midwestern regions of the United States.13 A person's risk of lung cancer can be calculated using a validated online tool available at http://www.diseaseriskindex.harvard.edu/update/index.htm.
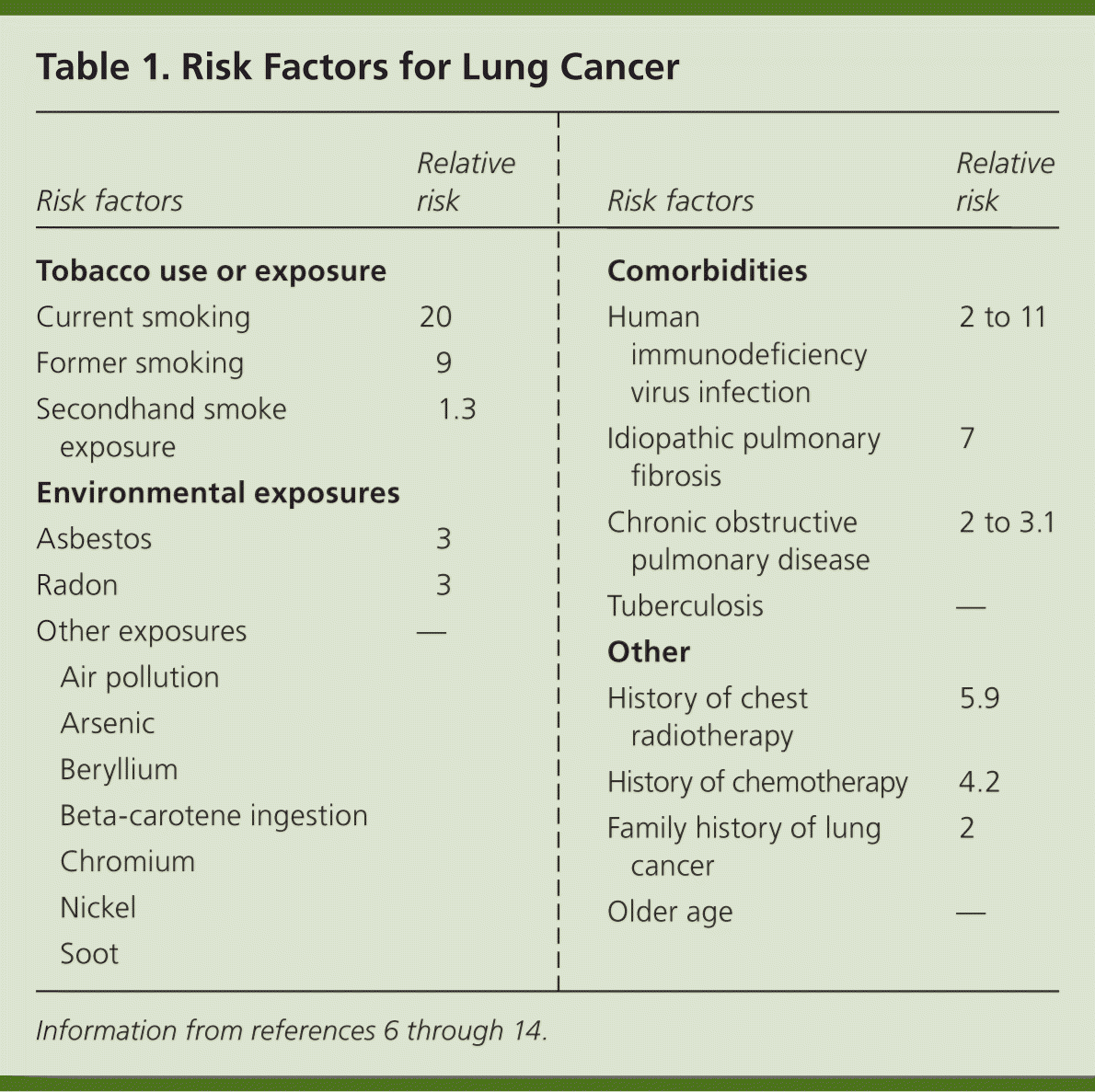
| Risk factors | Relative risk | |
|---|---|---|
| Tobacco use or exposure | ||
| Current smoking | 20 | |
| Former smoking | 9 | |
| Secondhand smoke exposure | 1.3 | |
| Environmental exposures | ||
| Asbestos | 3 | |
| Radon | 3 | |
| Other exposures | — | |
| Air pollution | ||
| Arsenic | ||
| Beryllium | ||
| Beta-carotene ingestion | ||
| Chromium | ||
| Nickel | ||
| Soot | ||
| Comorbidities | ||
| Human immunodeficiency virus infection | 2 to 11 | |
| Idiopathic pulmonary fibrosis | 7 | |
| Chronic obstructive pulmonary disease | 2 to 3.1 | |
| Tuberculosis | — | |
| Other | ||
| History of chest radiotherapy | 5.9 | |
| History of chemotherapy | 4.2 | |
| Family history of lung cancer | 2 | |
| Older age | — | |
Etiology
A combination of intrinsic factors and exposure to environmental carcinogens is involved in the pathogenesis of lung cancer.7 Preinvasive lesions such as adenocarcinoma in situ and minimally invasive adenocarcinoma are well described and show that there is likely a stepwise progression from dysplasia to malignancy.7 Familial and genetic variations can predispose a person to lung cancer, even nonsmokers.
Many genetic mutations within tumors have been identified. For example, mutations in the epidermal growth factor receptor (EGFR) gene are present in 20% of adenocarcinomas15; patients with this mutation are candidates for targeted molecular therapy with a drug that inhibits EGFR (erlotinib [Tarceva] or afatinib [Gilotrif]) or with a monoclonal antibody against EGFR (cetuximab [Erbitux]).15 Tumor mutations may also predict response to or toxicity from certain chemotherapies and are an important area for future investigation.15
Pathology
Lung cancer is classified by its histologic appearance into small cell lung cancer (SCLC) or non–small cell lung cancer (NSCLC; eTable A). NSCLC is divided into adenocarcinoma, squamous cell carcinoma, and large cell carcinoma; these are further subclassified.16 NSCLC is sometimes poorly differentiated and only distinguishable by immunohistochemical stains and molecular testing. This is problematic when only a small amount of tissue is available for testing. The optimal choice of treatment relies on a complete phenotypic and genotypic characterization of the tumor.
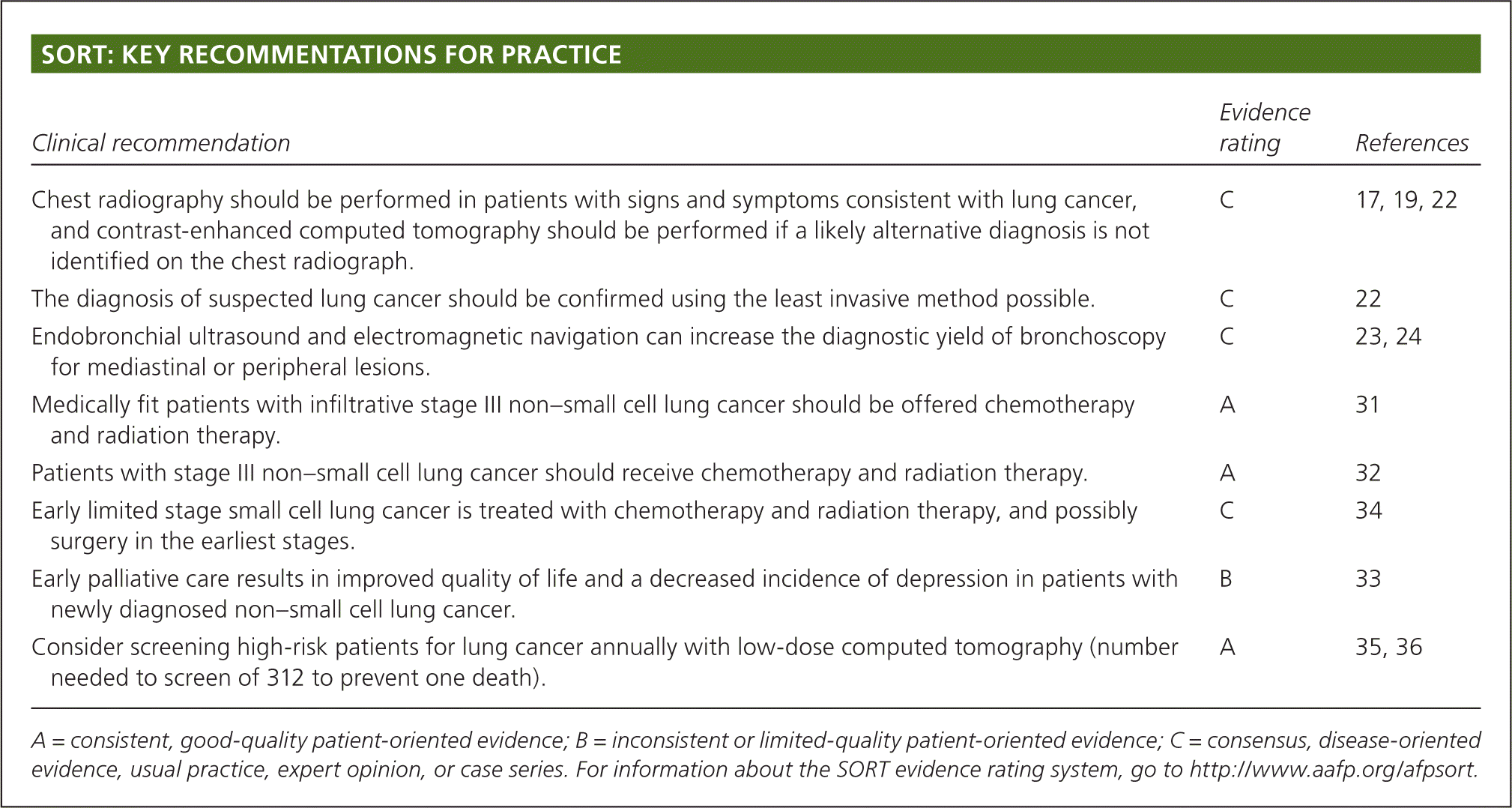
| Type | Percentage of all lung cancers | Classic presentation | |
|---|---|---|---|
| Non–small cell lung cancer | 80 | ||
| Adenocarcinoma (lepidic, acinar, papillary, micropapillary, fetal, colloid, mucinous) | 40 | Peripheral | |
| Squamous cell carcinoma | 25 | Central, associated more with smoking | |
| Large cell carcinoma | 10 | Peripheral | |
| Small cell lung cancer (small cell, combined small cell) | 15 | Central, massive lymphadenopathy, paraneoplastic syndromes | |
| Other uncommon types (such as carcinoid) | 5 | Varies | |
Clinical Presentation
Patients with lung cancer are almost always symptomatic at diagnosis.17 Symptoms can be caused by the primary tumor (e.g., cough, hemoptysis); intrathoracic spread (e.g., Horner syndrome, superior vena cava obstruction); and distant metastases (e.g., bone pain). Tables 218 and 317 summarize these symptoms. Symptoms can also be caused by paraneoplastic syndromes (Table 417 ), such as the syndrome of inappropriate antidiuretic hormone. These symptoms are a result of ectopic production of hormones from the tumor or the body's reaction to the tumor, and are not directly attributable to the tumor or metastasis. About 10% of patients with lung cancer present with a paraneoplastic syndrome, and this rate is higher in patients with SCLC.17 The best treatment for paraneoplastic syndromes is treatment of the underlying cancer.17 Digital clubbing is a common paraneoplastic syndrome finding that is poorly understood, and it is more common with NSCLC.
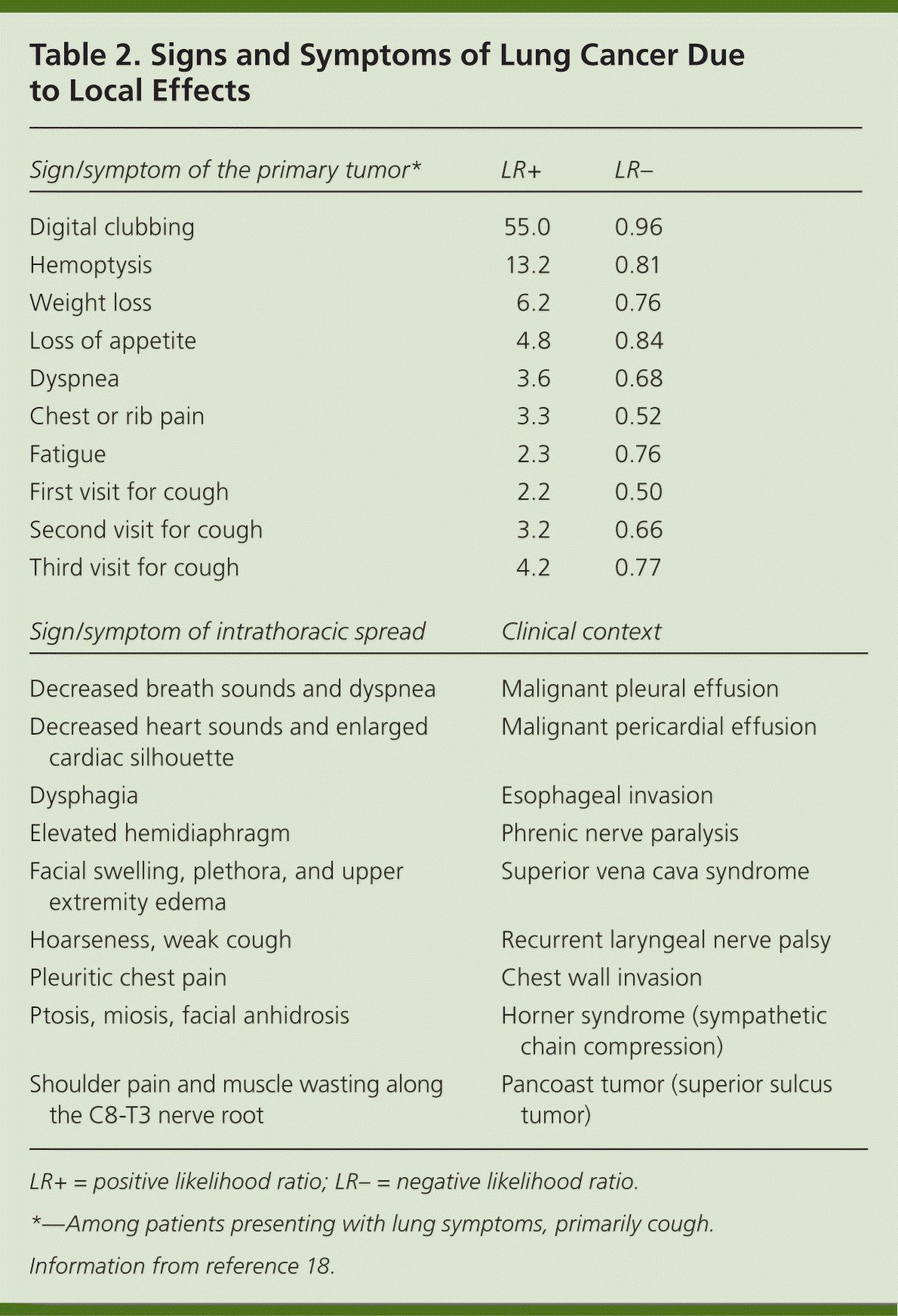
| Sign/symptom of the primary tumor* | LR+ | LR– |
|---|---|---|
| Digital clubbing | 55.0 | 0.96 |
| Hemoptysis | 13.2 | 0.81 |
| Weight loss | 6.2 | 0.76 |
| Loss of appetite | 4.8 | 0.84 |
| Dyspnea | 3.6 | 0.68 |
| Chest or rib pain | 3.3 | 0.52 |
| Fatigue | 2.3 | 0.76 |
| First visit for cough | 2.2 | 0.50 |
| Second visit for cough | 3.2 | 0.66 |
| Third visit for cough | 4.2 | 0.77 |
| Sign/symptom of intrathoracic spread | Clinical context | |
| Decreased breath sounds and dyspnea | Malignant pleural effusion | |
| Decreased heart sounds and enlarged cardiac silhouette | Malignant pericardial effusion | |
| Dysphagia | Esophageal invasion | |
| Elevated hemidiaphragm | Phrenic nerve paralysis | |
| Facial swelling, plethora, and upper extremity edema | Superior vena cava syndrome | |
| Hoarseness, weak cough | Recurrent laryngeal nerve palsy | |
| Pleuritic chest pain | Chest wall invasion | |
| Ptosis, miosis, facial anhidrosis | Horner syndrome (sympathetic chain compression) | |
| Shoulder pain and muscle wasting along the C8-T3 nerve root | Pancoast tumor (superior sulcus tumor) | |
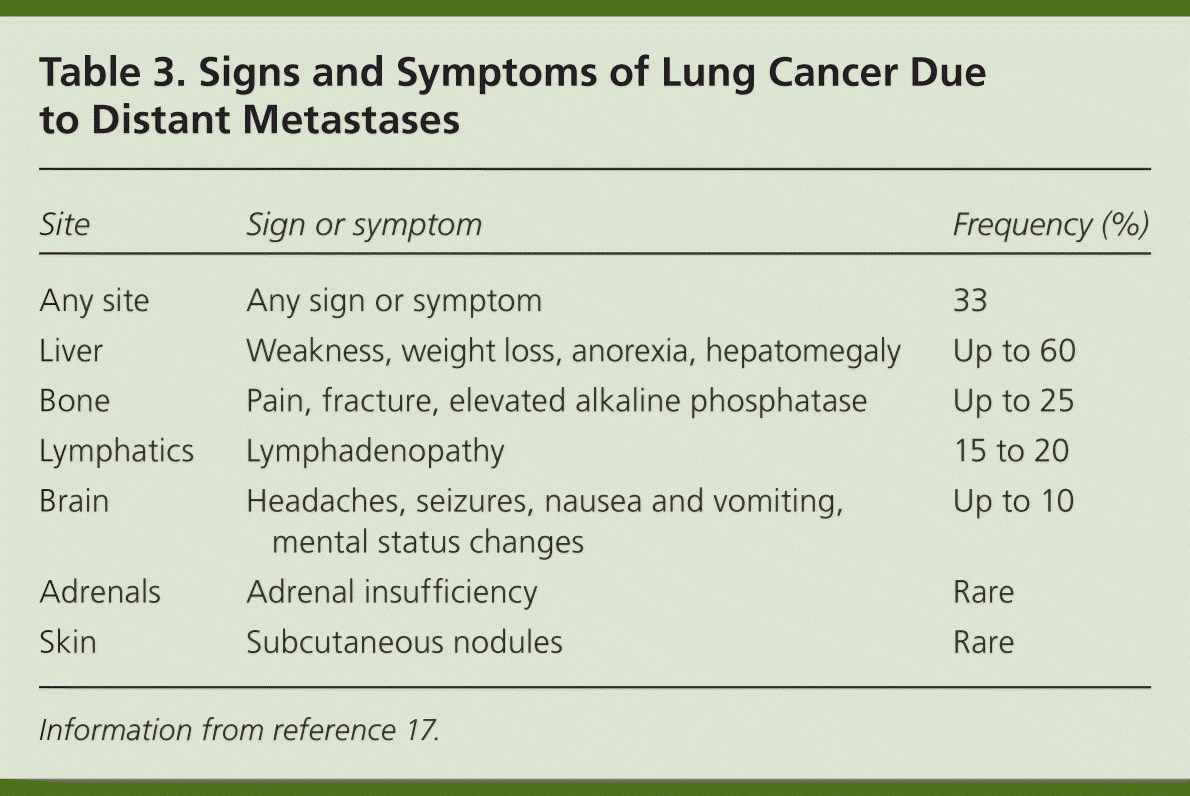
| Site | Sign or symptom | Frequency (%) |
|---|---|---|
| Any site | Any sign or symptom | 33 |
| Liver | Weakness, weight loss, anorexia, hepatomegaly | Up to 60 |
| Bone | Pain, fracture, elevated alkaline phosphatase | Up to 25 |
| Lymphatics | Lymphadenopathy | 15 to 20 |
| Brain | Headaches, seizures, nausea and vomiting, mental status changes | Up to 10 |
| Adrenals | Adrenal insufficiency | Rare |
| Skin | Subcutaneous nodules | Rare |
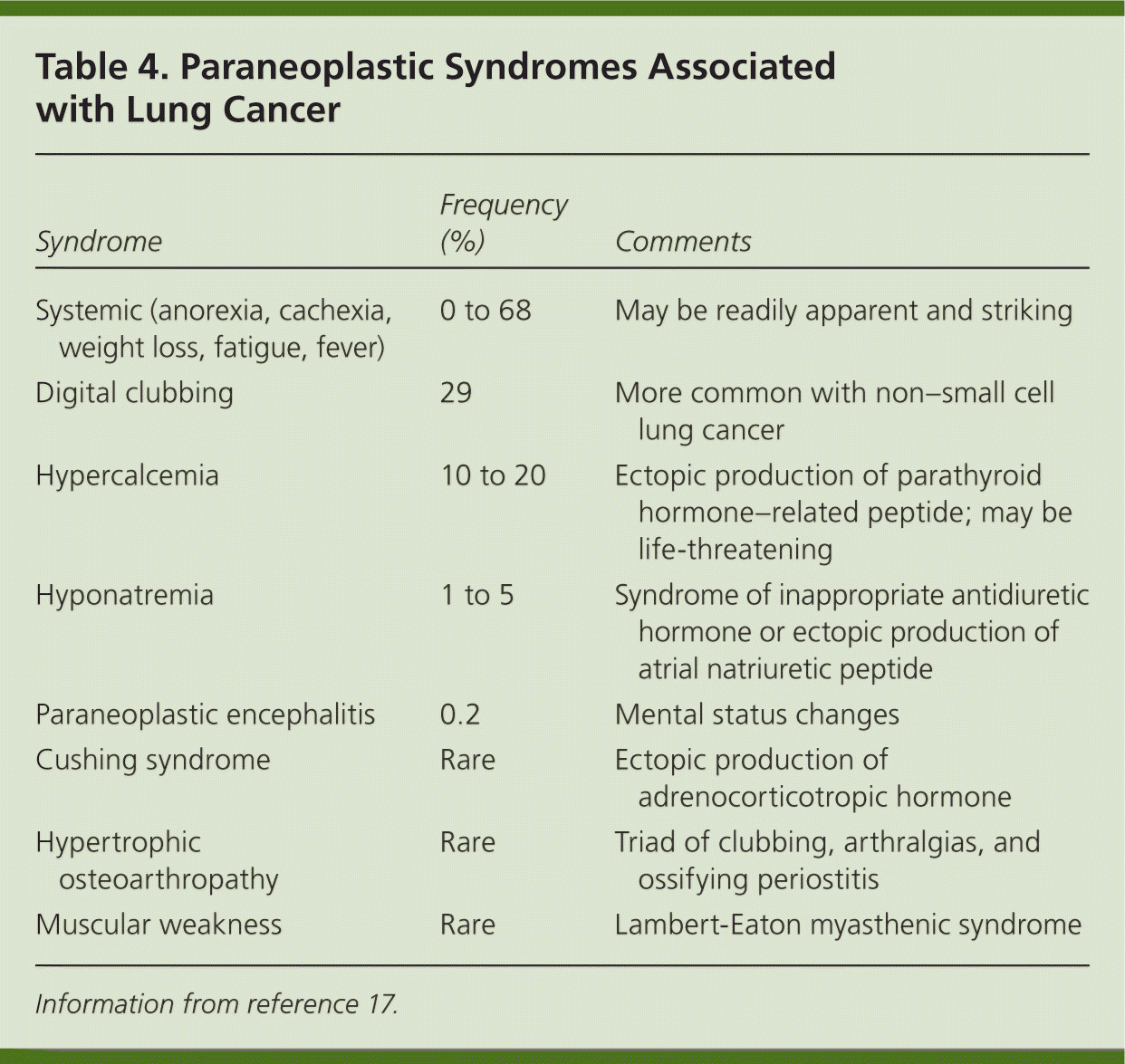
| Syndrome | Frequency (%) | Comments |
|---|---|---|
| Systemic (anorexia, cachexia, weight loss, fatigue, fever) | 0 to 68 | May be readily apparent and striking |
| Digital clubbing | 29 | More common with non–small cell lung cancer |
| Hypercalcemia | 10 to 20 | Ectopic production of parathyroid hormone–related peptide; may be life-threatening |
| Hyponatremia | 1 to 5 | Syndrome of inappropriate antidiuretic hormone or ectopic production of atrial natriuretic peptide |
| Paraneoplastic encephalitis | 0.2 | Mental status changes |
| Cushing syndrome | Rare | Ectopic production of adrenocorticotropic hormone |
| Hypertrophic osteoarthropathy | Rare | Triad of clubbing, arthralgias, and ossifying periostitis |
| Muscular weakness | Rare | Lambert-Eaton myasthenic syndrome |
Most data about symptoms at presentation of lung cancer are from referral centers, making extrapolation to the primary care setting difficult.19 Two individual symptoms that significantly increase the likelihood of lung cancer are digital clubbing and hemoptysis.18–21 Other independent predictors of lung cancer include loss of appetite, weight loss, fatigue, dyspnea, chest or rib pain, and an increasing number of visits to evaluate persistent cough.18 Patients rarely present with only one symptom, and the positive predictive value is higher when two or more symptoms are reported. For example, the combination of weight loss and hemoptysis has a positive predictive value of 9.2%.19 Lung cancer should be highly suspected in any patient older than 40 years with risk factors and symptoms. However, physicians must remember that lung cancer can occur in younger persons and in individuals without known risk factors.
Initial Evaluation
The initial evaluation of a patient with suspected lung cancer begins with a history and physical examination; complete blood count; measurement of alkaline phosphatase, hepatic transaminase, and calcium levels; chemistries (electrolytes, blood urea nitrogen, creatinine); and chest radiography.22 Normal findings on a chest radiograph do not rule out lung cancer because a small tumor can be hidden within the mediastinum or elsewhere in the chest. If suspicion remains high because a likely alternative diagnosis is not identified on the chest radiograph, contrast-enhanced computed tomography (CT) should be performed, followed by positron emission tomography if necessary.17,19,22
A multidisciplinary team consisting of a pulmonologist, medical oncologist, radiation oncologist, pathologist, radiologist, and thoracic surgeon then plans the diagnostic evaluation, the results of which guide treatment and determine prognosis. The patient's family physician should remain involved in the patient's care to ensure that the values and wishes of the patient and family are considered and, if necessary, to coordinate end-of-life care.
Diagnostic Evaluation
The diagnostic evaluation includes three simultaneous steps: tissue diagnosis, staging, and functional evaluation.
TISSUE DIAGNOSIS
Although experienced physicians can often diagnose the type of lung cancer based on clinical presentation and radiographic appearance, an adequate tissue sample is imperative to optimize the diagnosis and plan treatment.22 Molecular testing requires a significant amount of tissue. Targeted therapies can increase treatment options for patients with advanced disease or poor functional status. Molecular testing is also standard in never smokers with squamous cell tumors, making ample tissue all the more essential in such patients.22 For small or peripherally located lung cancers, this can be challenging.
A variety of diagnostic methods are available that yield cytology samples or small biopsies. The choice of procedure depends on the type, location, and size of the tumor; comorbidities; and accessibility of metastases (Table 5).22–24 In general, the least invasive method possible should be used.22 If the procedure fails to obtain tissue, a more invasive method is needed. Conventional bronchoscopy works best for central lesions, whereas CT-guided transthoracic needle aspiration is typically the first-line method for peripheral lesions. Endobronchial ultrasound24 and electromagnetic navigation23 are some of the newer procedures that may increase the diagnostic yield of bronchoscopy for select patients with mediastinal or peripheral lesions.24
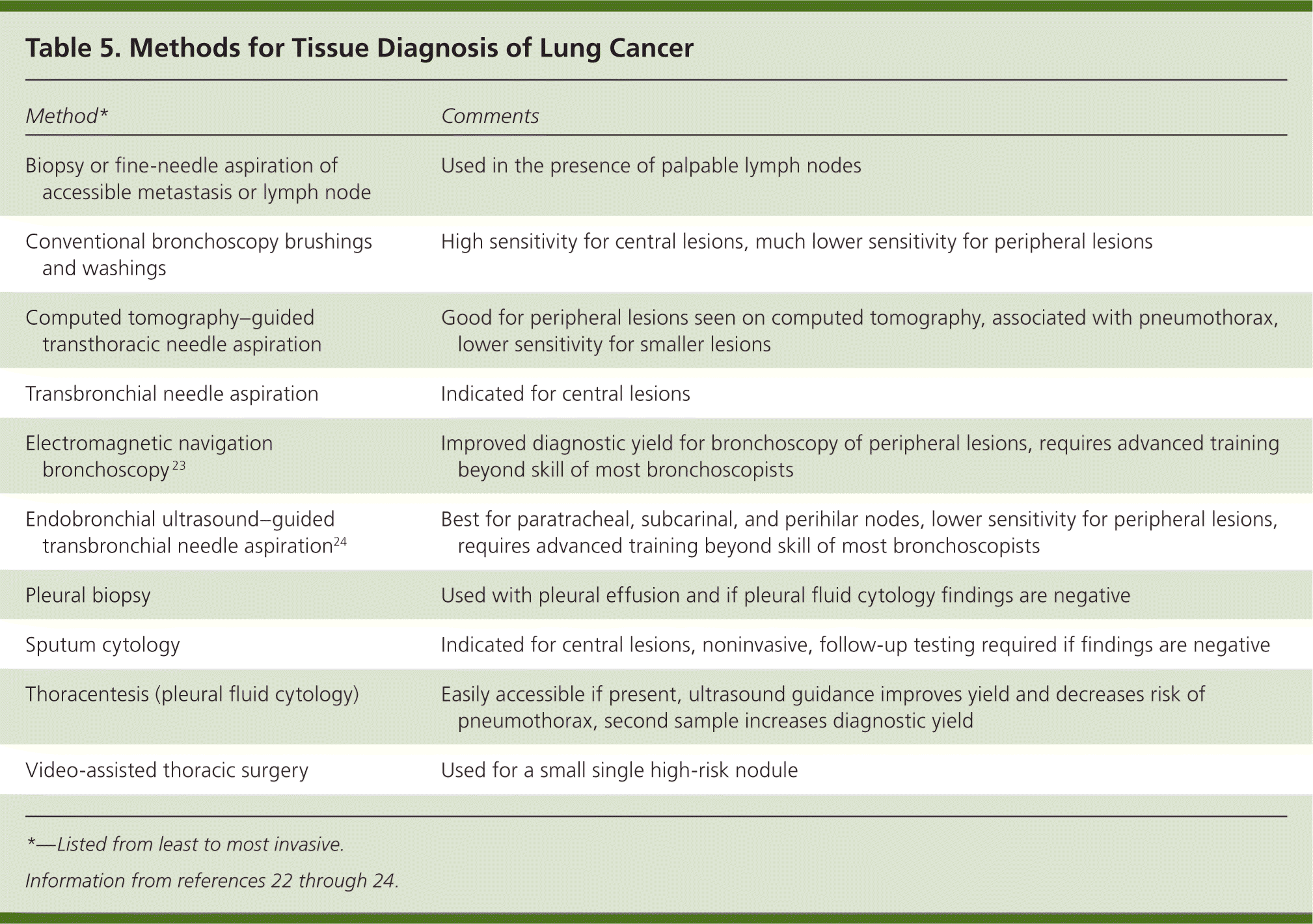
| Method* | Comments |
|---|---|
| Biopsy or fine-needle aspiration of accessible metastasis or lymph node | Used in the presence of palpable lymph nodes |
| Conventional bronchoscopy brushings and washings | High sensitivity for central lesions, much lower sensitivity for peripheral lesions |
| Computed tomography–guided transthoracic needle aspiration | Good for peripheral lesions seen on computed tomography, associated with pneumothorax, lower sensitivity for smaller lesions |
| Transbronchial needle aspiration | Indicated for central lesions |
| Electromagnetic navigation bronchoscopy 23 | Improved diagnostic yield for bronchoscopy of peripheral lesions, requires advanced training beyond skill of most bronchoscopists |
| Endobronchial ultrasound–guided transbronchial needle aspiration24 | Best for paratracheal, subcarinal, and perihilar nodes, lower sensitivity for peripheral lesions, requires advanced training beyond skill of most bronchoscopists |
| Pleural biopsy | Used with pleural effusion and if pleural fluid cytology findings are negative |
| Sputum cytology | Indicated for central lesions, noninvasive, follow-up testing required if findings are negative |
| Thoracentesis (pleural fluid cytology) | Easily accessible if present, ultrasound guidance improves yield and decreases risk of pneumothorax, second sample increases diagnostic yield |
| Video-assisted thoracic surgery | Used for a small single high-risk nodule |
STAGING
Clinical staging is based on all information obtained before treatment, including findings from CT and positron emission tomography and invasive staging such as mediastinoscopy.25 Pathologic staging is performed after surgical resection and may upgrade or downgrade the clinical staging. NSCLC is staged according to the TNM (tumor size, nodes, metastasis) system. The 7th edition of this system, which is the most recent, is based on a retrospective analysis of more than 81,000 cases of lung cancer collected from around the world between 1990 and 2000.26,27 An online calculator available at http://staginglungcancer.org/calculator summarizes the TNM staging system and provides corresponding drawings, CT scans, and survival curves.
For SCLC, American College of Chest Physicians (ACCP) guidelines recommend using the 7th edition TNM staging system for prognosis and placement into clinical trials.25,27 Many physicians use the simpler Veterans Administration Lung Study Group classification system to stage SCLC for treatment purposes.28 Limited disease is cancer confined within a single tolerable radiation field. Extensive disease is cancer that has extended outside of a single hemithorax.
FUNCTIONAL CAPACITY
Patients with advanced age, poor nutritional status, or multiple comorbidities may not be able to tolerate lung resection, radiation, or chemotherapy and thus treatment must be individualized. The Eastern Cooperative Oncology Group Performance Status is an easy-to-use grading tool to predict how well patients will tolerate chemotherapy 29:
0: Fully active, able to carry on predisease activity without restriction
1: Restricted in physically strenuous activity but ambulatory and able to perform light or sedentary work (e.g., light housework, office work)
2: Ambulatory and capable of all self-care but unable to carry out any work activities, up and about more than 50% of waking hours
3: Capable of only limited self-care, confined to a bed or chair more than 50% of waking hours
4: Completely disabled, incapable of any self-care, totally confined to a bed or chair
5: Dead
Candidates for lung resection need standard preoperative evaluation plus pulmonary function testing and carbon monoxide diffusion in the lung measurements to estimate postsurgical lung reserve.30 Brain magnetic resonance imaging is standard in the pretreatment evaluation, except in patients with stage IA NSCLC.30
Treatment
NON–SMALL CELL LUNG CANCER
The treatment of NSCLC is well detailed in the 2013 ACCP evidence-based practice guidelines.31–33 The nuances of treatment are evolving, complex, and largely beyond the scope of this review, yet a few themes are significant. Morbidity and mortality outcomes may be improved for patients evaluated and treated by a surgical thoracic oncologist in conjunction with a multidisciplinary team at a lung cancer treatment center.
Surgical resection is indicated in medically fit patients with resectable stage I or II NSCLC, preferably a minimally invasive approach such as video-assisted thoracic surgery.31 The goal for stage III infiltrative NSCLC is eradicating known intrathoracic cancer while diminishing subsequent intrathoracic and systemic disease,32 usually through chemotherapy and radiation based on tumor histology and the patient's functional status. In stage IV tumors, multidisciplinary management options are also largely dictated by histology and patient status.
Palliative care should be initiated early in patients with stage IV NSCLC, or at any stage if underlying morbidity or patient choice prevents intent-to-cure therapy. Early palliative care significantly improves quality of life, decreases the incidence of depression in patients with newly diagnosed NSCLC, and may prolong survival.33 Additional management decisions may be influenced by a patient's involvement in an approved clinical trial.
SMALL CELL LUNG CANCER
Limited stage SCLC is treated with an intent to cure; treatment results in a five-year survival rate of up to 25%.34 For early limited stage SCLC, surgery may be indicated. For both limited stage and extensive stage SCLC, concurrent chemotherapy and radiation therapy with a platinum-based agent and at least one other chemotherapeutic agent should be pursued. The five-year survival rate is virtually zero for extensive stage SCLC. As with more extensive NSCLC, a patient's comorbidities, the extent of disease, and patient preferences are integral to making treatment decisions, and palliative care should be initiated early.
Prognosis
Prognosis is better if presenting symptoms are caused by the primary tumor rather than by metastatic disease or paraneoplastic syndromes.17 It is also better in earlier stages of cancer. Survival rates at five years can be greater than 50% for those with localized disease, but decrease to less than 5% in those with distant disease.4 The online staging calculator at http://staginglungcancer.org/calculator can also be used for prognosis.
Screening
The U.S. Preventive Services Task Force (USPSTF) supports annual low-dose CT to screen for lung cancer in patients 55 to 80 years of age with at least a 30 pack-year history who currently smoke or have quit within the past 15 years.35 The USPSTF cites the National Lung Screening Trial, which found a number needed to screen of 312 to prevent one lung cancer death in five years with three screening examinations.36 The recommendation was also based on extensive modeling studies to refine estimates of benefit and harm.37 The American Academy of Family Physicians concludes that the evidence is insufficient to recommend for or against low-dose CT screening for lung cancer.38 This conclusion is based on the fact that the National Lung Screening Trial was performed at major centers with strict protocols (not community hospitals) and 40% of patients required some type of follow-up study or intervention because of positive results, and that the long-term hazards from cumulative radiation exposure with this screening are unknown. In light of differing guidelines, an approach of shared decision making and educating patients on the potential benefits and risks in relation to their personal health and health care setting is essential.
Prevention
Never smoking is the best way to prevent lung cancer, and smoking cessation is helpful. The USPSTF recommends screening every patient for tobacco use and encouraging smoking cessation for smokers at every appointment.39 Physician counseling techniques can be effective when tailored to a patient's willingness to change.40 A variety of pharmacologic modalities are available that work best when combined with social and behavioral support. Legislation such as smoking bans in public buildings, prohibiting marketing of tobacco products to minors, and taxation of tobacco products likely play a role in decreasing tobacco use.41
Physician and Patient Resources
The National Cancer Institute website (http://www.cancer.gov/cancertopics/pdq/adulttreatment) is a helpful resource for physicians and patients. Clinical practice guidelines are summarized on the National Comprehensive Cancer Network website (http://www.nccn.org/professionals/physician_gls/f_guidelines.asp#site; free registration required).
Data Sources: We searched PubMed, Clinical Inquiries, the Cochrane database, the USPSTF, and Ovid. In addition, we relied heavily on the ACCP 2013 lung cancer evidence-based guidelines. We used references from recent review articles from UptoDate and American Family Physician. We limited our search timeline to the previous five years. Search dates: January through March 2014.
The views expressed are the authors' and do not reflect the official policy or position of the U.S. government, Department of the Navy, or Department of Defense.