
Am Fam Physician. 2017;95(6):393-394
Author disclosure: No relevant financial affiliations.
Elbasvir/grazoprevir (Zepatier) is a fixed-dose combination of a hepatitis C virus (HCV) NS5A inhibitor and HCV NS3/4A protease inhibitor. It is labeled for the treatment of chronic HCV genotype 1 or 4 infection in treatment-naive or treatment-experienced adults with or without compensated cirrhosis. It is also labeled for use in patients coinfected with human immunodeficiency virus 1 (HIV-1).
SAFETY
Clinically significant reactions are rare with elbasvir/grazoprevir treatment. In studies of approximately 1,700 patients, 1% of patients had an alanine aminotransferase level greater than five times the upper limit of normal.1 Increases in alanine aminotransferase levels were usually asymptomatic and occurred at or after two months of treatment. Most cases resolved during or at completion of therapy. Elbasvir/grazoprevir should not be used in patients with moderate to severe hepatic impairment (Child-Pugh score of B or C). No dosage adjustments are necessary for patients with any level of renal impairment, including those on hemodialysis.
Elbasvir/grazoprevir is metabolized in the liver via the cytochrome P450 3A enzyme system and can be affected by the many medications that induce or inhibit this system. Carbamazepine (Tegretol), efavirenz (Sustiva), phenytoin (Dilantin), rifampin, and St. John's wort will dramatically decrease plasma concentrations of both elbasvir and grazoprevir. Cyclosporine (Sandimmune) and some protease-inhibitor HIV medications will significantly raise plasma concentrations of grazoprevir, increasing the risk of liver damage. Use of elbasvir/grazoprevir has not been studied in pregnancy, and it should not be used by pregnant women. It is unknown whether elbasvir/grazoprevir is excreted in breast milk.1
TOLERABILITY
EFFECTIVENESS
Two placebo-controlled studies of 656 total patients showed that elbasvir/grazoprevir produces a response rate (i.e., sustained virologic response four months after treatment) in 95% to 99% of patients.2,3 Treatment-naive patients with HCV genotype 1a have a slightly lower response rate (92%). Response rates are higher in treatment-naive patients with genotype 1b (99%) or genotype 4 (100%), although the study size was smaller in the latter group.2 Response rates are lowest (70%) in patients with NS5A resistance–associated polymorphisms of genotype 1a, which are present in about 12% of patients in the United States. Patients with this characteristic should also be treated with ribavirin, which increases the response rate to 100%.1 There are no effectiveness studies comparing elbasvir/grazoprevir with other new long-term HCV medications.
PRICE
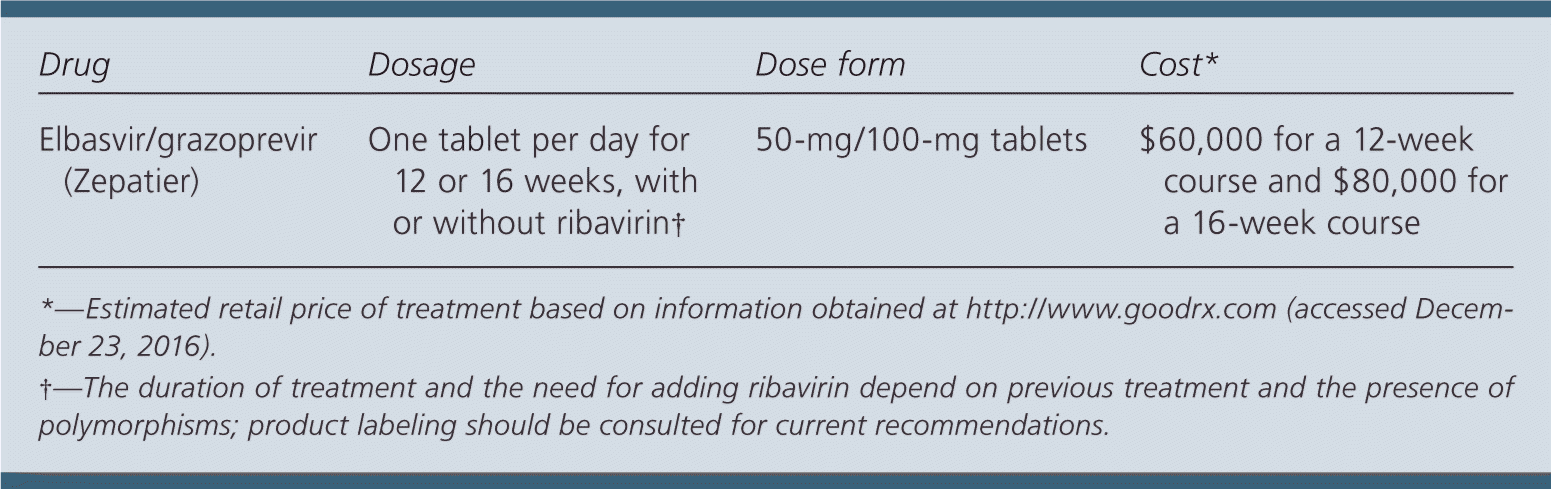
Elbasvir/grazoprevir costs approximately $60,000 for a 12-week course and $80,000 for a 16-week course. Comparatively, a 12-week course of sofosbuvir/velpatasvir (Epclusa) costs approximately $73,000, and an eight-week course of ledipasvir/sofosbuvir (Harvoni) costs approximately $65,000.
SIMPLICITY
Elbasvir/grazoprevir, 50 mg/100 mg, is taken orally once per day with or without food for 12 or 16 weeks. Patients with HCV genotype 1a should be tested for the presence of NS5A resistance–associated polymorphisms before initiation of treatment. Potential drug interactions with existing treatments should also be assessed before beginning therapy. The duration of treatment and the need for adding ribavirin depend on previous treatment and the presence of polymorphisms, and the product labeling should be consulted for current recommendations. Serum hepatic function should be tested before starting therapy and at week 8 of a 12-week treatment or at week 12 of a 16-week treatment. Stopping treatment should be considered if alanine aminotransferase levels are persistently above 10 times the upper limit of normal. Viral load should be checked 12 weeks after cessation of treatment to confirm response.
Bottom Line
Elbasvir/grazoprevir is a safe and effective treatment for HCV genotypes 1 and 4, with or without compensated cirrhosis or HIV-1 coinfection. Patients with renal impairment, including those on hemodialysis, can use the medication. Treatment regimens vary based on whether the patient has had previous treatment and the presence of NS5A resistance–associated polymorphisms. Physicians should be aware of possible drug interactions before adding or changing medications in a patient taking elbasvir/grazoprevir.
