Am Fam Physician. 2017;96(11):online
Author disclosure: No relevant financial affiliations.

| Benefits | Harms |
|---|---|
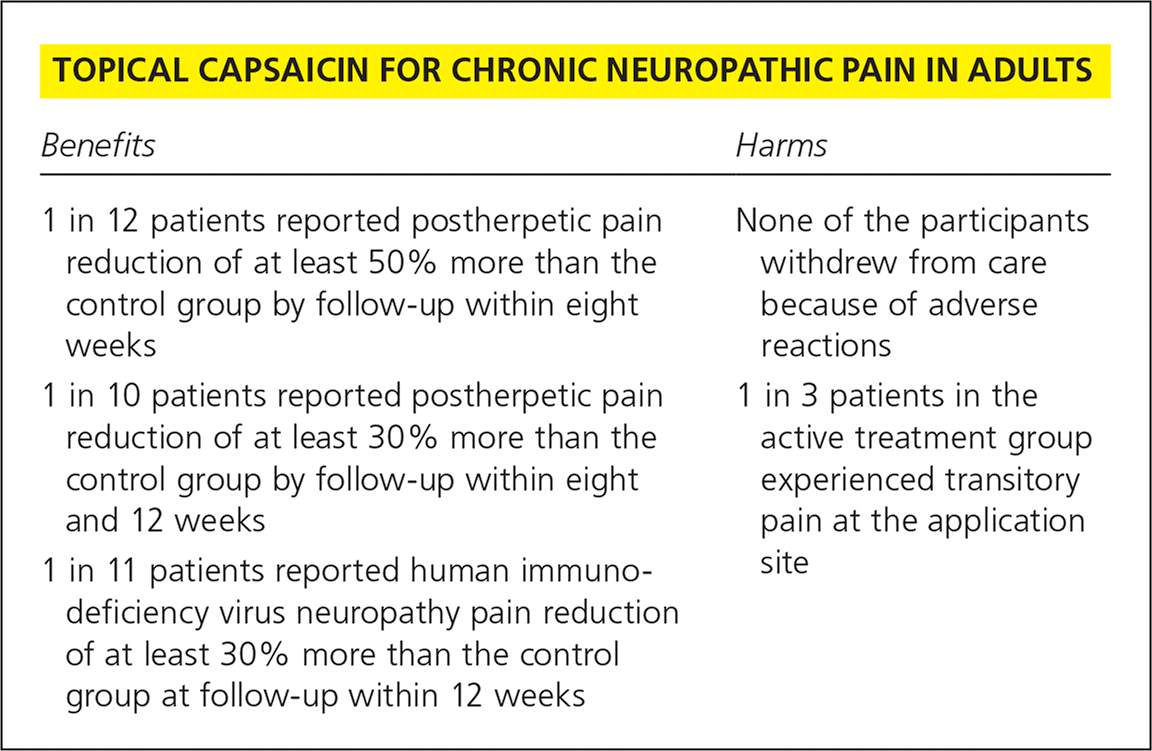
| 1 in 12 patients reported postherpetic pain reduction of at least 50% more than the control group by follow-up within eight weeks | None of the participants withdrew from care because of adverse reactions |
| 1 in 10 patients reported postherpetic pain reduction of at least 30% more than the control group by follow-up within eight and 12 weeks | 1 in 3 patients in the active treatment group experienced transitory pain at the application site |
| 1 in 11 patients reported human immuno-deficiency virus neuropathy pain reduction of at least 30% more than the control group at follow-up within 12 weeks |
Details for This Review
Study Population: Patients 16 years or older with neuropathic pain of any origin for more than 12 weeks
Efficacy End Points: Patients reported postherpetic pain reduction of at least 50% more than the control group by follow-up within eight weeks; patients reported postherpetic pain reduction of at least 30% more than the control group by follow-up within eight and 12 weeks; and patients reported human immunodeficiency virus neuropathy pain reduction of at least 30% more than the control group at follow-up within 12 weeks.
Harm End Points: No additional patients withdrew from care because of adverse reactions. One in three patients in the active treatment group experienced transitory pain at the application site.
Narrative: Chronic neuropathic pain is a common, sometimes debilitating, condition that can result from a multitude of origins. Currently, pregabalin (Lyrica) is the only approved medication option. Physicians commonly prescribe off-label use of selective serotonin reuptake inhibitors, gabapentin (Neurontin), tricyclic antidepressants, and antiepileptics. This review assesses the effectiveness and duration of a single application of high-concentration (8%) topical capsaicin for pain relief in patients with neuropathic pain lasting more than eight weeks.
A 2017 Cochrane review of eight double-blind, randomized controlled studies included 2,488 patients, and compared the effect of a single topical application of high-concentration (8%) capsaicin for long-term neuropathic pain relief compared with a control group.1 A 0.04% concentration capsaicin control was used in all studies to blind participants to the burning and erythema experienced after application. Pain was not assessed in the first posttreatment week because capsaicin preparations often cause localized pain at the application site for several days.
For patients with postherpetic neuropathic pain, topical capsaicin provided moderate pain relief (30% to 50% reduction from baseline) with a number needed to treat (NNT) of 12 (29% vs. 20% in high-concentration application and control groups, respectively) to reduce pain by 50% at follow-up within eight weeks, and an NNT of 10 (43% vs. 34% in high-concentration application and control groups, respectively) to reduce pain by at least 30% within eight weeks. Three studies also looked at 30% pain reduction within 12 weeks and found a similar benefit, with an NNT of 10 (46% vs 37% in high-concentration application and control groups, respectively).
This review also included two studies that found at least a 30% reduction in pain in patients with human immunodeficiency virus neuropathy pain treated with a single application of high-concentration capsaicin with an NNT of 11 at follow-up within 12 weeks (39% vs. 30% in high-concentration application and control groups, respectively). These studies further differentiated effects on pain with a 30-minute total application time vs. 60-minute total application time, which did not show a meaningful difference in outcomes. The 30-minute application of high-concentration capsaicin had a relative risk (RR) of 2.95 (95% confidence interval [CI], 0.73 to 11.88) compared with the 60-minute application of high-concentration capsaicin, which had an RR of 1.34 (95% CI, 1.03 to 1.75).
Effects on diabetic peripheral neuropathy and neuropathic pain following inguinal herniorrhaphy were also reviewed; however, data in these cohorts were sparse. High-concentration topical capsaicin did not improve these conditions.
The most common adverse events were erythema and pain over the application site compared with the control group; however, these effects were transitory and minimal for almost all participants. There were 12 withdrawals because of adverse reactions in 1,507 participants treated with high-concentration capsaicin compared with nine withdrawals in 980 participants in the control group (number needed to harm was not calculated).
Caveats: Although the studies included 2,488 participants, the quality of evidence for each trial was very low to moderate given wide CIs, sparse data, and susceptibility to publication bias. About 50% of patients (n = 1,272) in this meta-analysis had postherpetic neuropathy. Improved heterogeneity in study populations to include more participants with other etiologies of neuropathic pain would allow for data to be generalized over a greater population. Additionally, better quality studies are needed to demonstrate definitive improvement.
Based on low-quality evidence, high-concentration capsaicin patches reduce neuropathic pain from herpes and human immunodeficiency virus infection by 30% to 50% compared with the low-concentration capsaicin control group.