
Am Fam Physician. 2018;97(9):online
Author disclosure: No relevant financial affiliations.
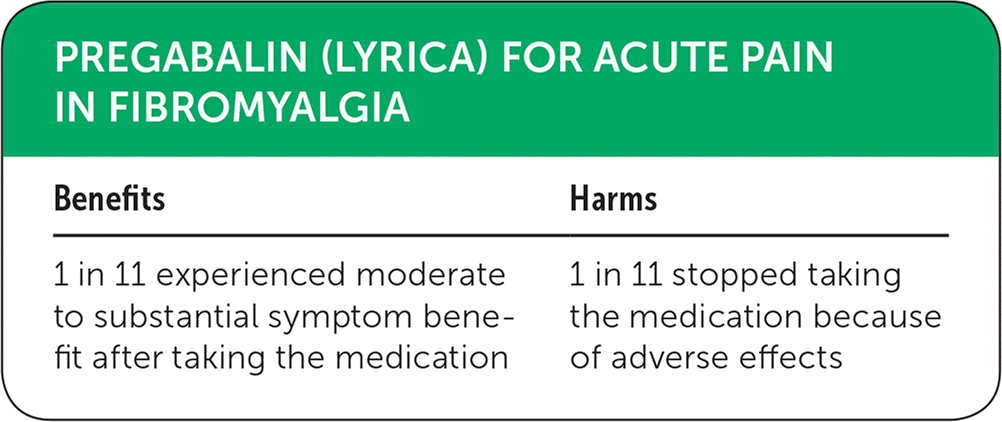
| Benefits | Harms |
|---|---|
| 1 in 11 experienced moderate to substantial symptom benefit after taking the medication | 1 in 11 stopped taking the medication because of adverse effects |
Details for This Review
Study Population: Adults with fibromyalgia
Efficacy End Points: At least a moderate to substantial symptom benefit
Harm End Points: Withdrawal from trials because of adverse effects
Narrative: Fibromyalgia is a potentially debilitating disease characterized by widespread pain, fatigue, and sleep problems. In its most severe form, fibromyalgia can be incapacitating. High-quality studies analyzed in a Cochrane review consistently showed pregabalin (Lyrica) to have an effect on reducing pain in approximately 9% of patients with fibromyalgia, with a number needed to treat of 11.1 The studies showed a moderate to substantial symptom benefit in 331 (36%) of 932 patients receiving pregabalin compared with 251 (27%) of 937 patients receiving a placebo. Symptom improvement is an important end point that matters to patients and can have a positive effect on quality of life.1
The studies also showed a number of adverse effects with pregabalin use, most commonly dizziness and somnolence. Adverse effects occurred in 17% of patients in the treatment group compared with 9% of patients in the placebo group. Patients had to discontinue taking the medication because of these adverse effects at about the same rate at which their symptoms improved, giving the medication a number needed to harm of 11. Given the benefit shown in multiple high-quality studies, we have labeled this treatment as green. However, patients should be made aware that they are equally as likely to experience intolerable adverse effects from the drug as they are to experience substantial benefit.
Caveats: The methodologic quality of the trials included in the Cochrane review was high. However, the studies did not assess the long-term tolerability of the medication. Most studies followed patients for 12 to 13 weeks (maximum of six months). This could be particularly important if the drug effectiveness decreases over time.
One notable caveat is the potential for functional unblinding of patients allocated to the pregabalin arm in these trials. Although 9% of patients withdrew because of adverse effects, there may have been other patients who experienced common adverse effects not serious enough to cause termination of participation in the trials. For example, according to data from the U.S. Food and Drug Administration,2 somnolence occurred in 20% of patients treated with pregabalin, compared with 4% of participants receiving placebo. Similarly, 38% of patients allocated to receive pregabalin experienced dizziness compared with only 9% of patients receiving placebo. Patients experiencing adverse effects were more likely to have been in the treatment group, and unblinding may have been more likely in this group. There was no difference in the effectiveness of pregabalin with nighttime dosing or a divided twice-a-day regimen.
Pregabalin provides good pain relief to some patients with fibromyalgia but does not work for most persons. Physicians should consider switching patients to other medications after a trial of pregabalin, if the desired treatment effect has not been achieved.
Copyright 2018 The NNT Group (theNNT.com). Used with permission.
This series is coordinated by Dean A. Seehusen, MD, MPH, AFP Assistant Medical Editor, and Daniel Runde, MD, from the NNT Group.
A collection of Medicine by the Numbers published in AFP is available at https://www.aafp.org/afp/mbtn.
This review is available from the NNT Group at http://www.thennt.com/nnt/pregabalin-lyrica-for-acute-fibromyalgia-pain/.
