
Am Fam Physician. 2018;97(11):741-748
Author disclosure: No relevant financial affiliations.
Most oncologic emergencies can be classified as metabolic, hematologic, structural, or treatment related. Tumor lysis syndrome is a metabolic emergency that presents as severe electrolyte abnormalities. Stabilization is focused on vigorous rehydration, maintaining urine output, and lowering uric acid levels. Hypercalcemia of malignancy, which is associated with poor outcomes, is treated with aggressive rehydration, intravenous bisphosphonates, and subspecialty consultation. Syndrome of inappropriate antidiuretic hormone should be suspected if a patient with cancer has hyponatremia. This metabolic condition is treated with fluid restriction or hypertonic saline, depending on the speed of development. Febrile neutropenia is one of the most common complications related to cancer treatment, particularly chemotherapy. It usually requires inpatient therapy with rapid administration of empiric antibiotics. Hyperviscosity syndrome may present as spontaneous bleeding and neurologic deficits, and is usually associated with Waldenström macroglobulinemia. Treatment includes plasmapheresis followed by targeted chemotherapy. Structural oncologic emergencies are caused by direct compression of nontumor structures by metastatic disease. Superior vena cava syndrome presents as facial edema with development of collateral venous circulation. Intravascular stenting leads to superior patient outcomes and is used in addition to oncology-directed chemotherapy and radiation therapy. Malignant epidural spinal cord compression is managed in conjunction with neurosurgery, but it is classically treated using steroids and/or surgery and radiation therapy. Malignant pericardial effusion may be treated with pericardiocentesis or a more permanent surgical intervention. Complications of cancer treatment are becoming more varied because of the use of standard and newer immunologic therapies. Palliative care is increasingly appropriate as a part of the team approach for treating patients with cancer.
The National Cancer Institute estimates that 14.5 million persons in the United States have cancer, and that number could reach 19 million by 2024.1 Family physicians should be familiar with the most prevalent oncologic emergencies because stabilization is often necessary, in addition to referrals for managing the underlying malignancy and initiating palliative measures.2 Some oncologic emergencies are insidious and take months to develop, whereas others manifest over hours, causing devastating outcomes such as paralysis and death.3 In many patients, cancer is not diagnosed until a related condition emerges. A patient-focused approach that includes education; cancer-specific monitoring; and team-based care, including palliative care, with continuous communication is recommended.4 Most oncologic emergencies can be categorized as metabolic, hematologic, structural, or treatment related (Table 15).
| Clinical recommendation | Evidence rating | References |
|---|---|---|
| Concurrent palliative care consultation should be offered to patients with cancer at the time of diagnosis. | C | 4 |
| Emergent use of antibiotics in patients with cancer who present with febrile neutropenia improves survival rates. | B | 22 |
| New-onset back pain in patients with cancer should be evaluated as epidural spinal cord compression until it is ruled out. | C | 3, 26 |
| More permanent surgical solutions for management of malignant pericardial effusions, such as pericardial windows and indwelling pericardial catheters, are associated with improved patient outcomes compared with percutaneous pericardiocentesis alone. | B | 29–31 |
| Complications from newer immunotherapy treatments often present as nonspecific and vague symptoms, such as flulike illness and rash, requiring a high level of suspicion in patients undergoing cancer treatment. | C | 40–42 |
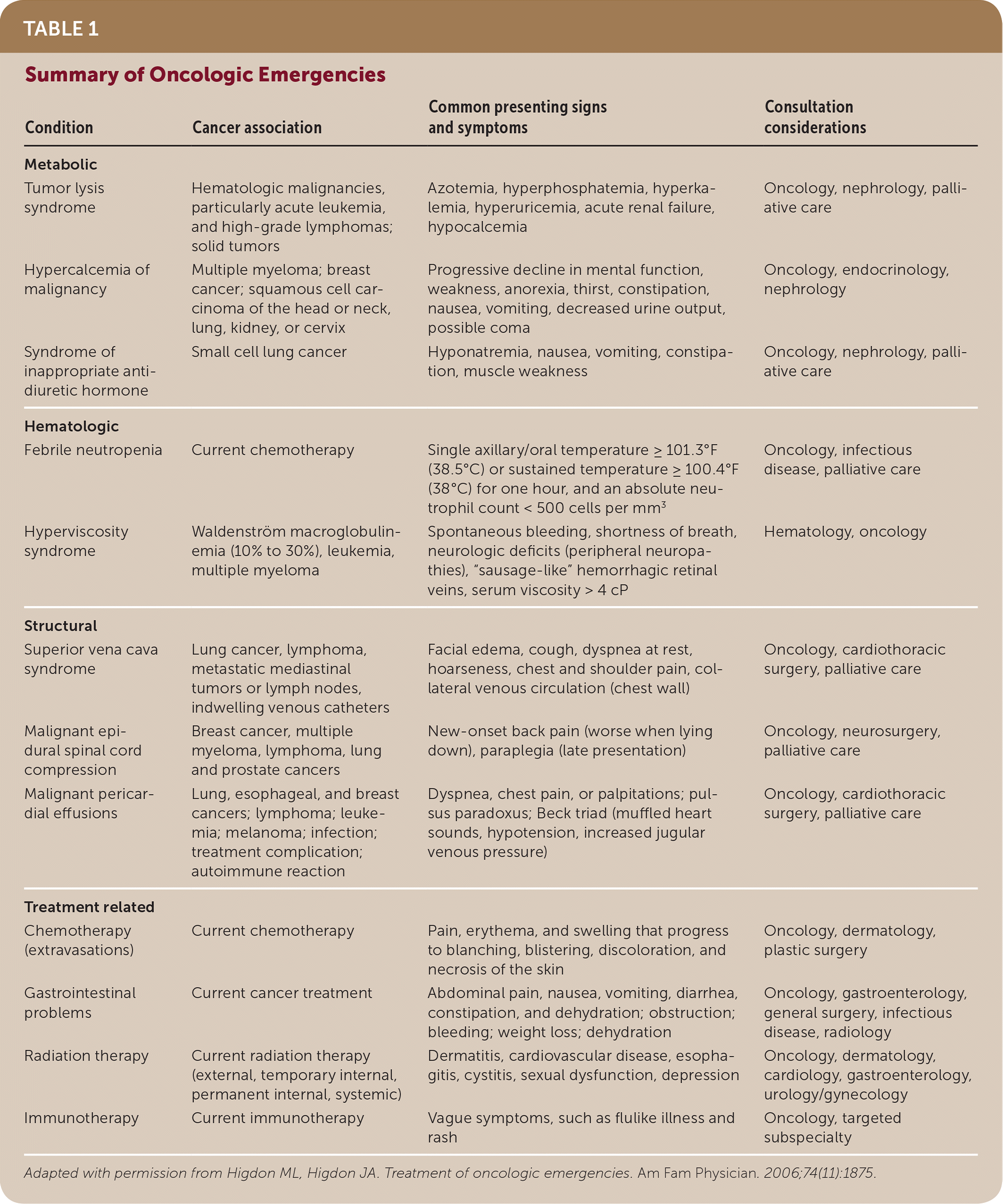
| Condition | Cancer association | Common presenting signs and symptoms | Consultation considerations |
|---|---|---|---|
| Metabolic | |||
| Tumor lysis syndrome | Hematologic malignancies, particularly acute leukemia, and high-grade lymphomas; solid tumors | Azotemia, hyperphosphatemia, hyperkalemia, hyperuricemia, acute renal failure, hypocalcemia | Oncology, nephrology, palliative care |
| Hypercalcemia of malignancy | Multiple myeloma; breast cancer; squamous cell carcinoma of the head or neck, lung, kidney, or cervix | Progressive decline in mental function, weakness, anorexia, thirst, constipation, nausea, vomiting, decreased urine output, possible coma | Oncology, endocrinology, nephrology |
| Syndrome of inappropriate antidiuretic hormone | Small cell lung cancer | Hyponatremia, nausea, vomiting, constipation, muscle weakness | Oncology, nephrology, palliative care |
| Hematologic | |||
| Febrile neutropenia | Current chemotherapy | Single axillary/oral temperature ≥ 101.3°F (38.5°C) or sustained temperature ≥ 100.4°F (38°C) for one hour, and an absolute neutrophil count < 500 cells per mm3 | Oncology, infectious disease, palliative care |
| Hyperviscosity syndrome | Waldenström macroglobulinemia (10% to 30%), leukemia, multiple myeloma | Spontaneous bleeding, shortness of breath, neurologic deficits (peripheral neuropathies), “sausage-like” hemorrhagic retinal veins, serum viscosity > 4 cP | Hematology, oncology |
| Structural | |||
| Superior vena cava syndrome | Lung cancer, lymphoma, metastatic mediastinal tumors or lymph nodes, indwelling venous catheters | Facial edema, cough, dyspnea at rest, hoarseness, chest and shoulder pain, collateral venous circulation (chest wall) | Oncology, cardiothoracic surgery, palliative care |
| Malignant epidural spinal cord compression | Breast cancer, multiple myeloma, lymphoma, lung and prostate cancers | New-onset back pain (worse when lying down), paraplegia (late presentation) | Oncology, neurosurgery, palliative care |
| Malignant pericardial effusions | Lung, esophageal, and breast cancers; lymphoma; leukemia; melanoma; infection; treatment complication; autoimmune reaction | Dyspnea, chest pain, or palpitations; pulsus paradoxus; Beck triad (muffled heart sounds, hypotension, increased jugular venous pressure) | Oncology, cardiothoracic surgery, palliative care |
| Treatment related | |||
| Chemotherapy (extravasations) | Current chemotherapy | Pain, erythema, and swelling that progress to blanching, blistering, discoloration, and necrosis of the skin | Oncology, dermatology, plastic surgery |
| Gastrointestinal problems | Current cancer treatment | Abdominal pain, nausea, vomiting, diarrhea, constipation, and dehydration; obstruction; bleeding; weight loss; dehydration | Oncology, gastroenterology, general surgery, infectious disease, radiology |
| Radiation therapy | Current radiation therapy (external, temporary internal, permanent internal, systemic) | Dermatitis, cardiovascular disease, esophagitis, cystitis, sexual dysfunction, depression | Oncology, dermatology, cardiology, gastroenterology, urology/gynecology |
| Immunotherapy | Current immunotherapy | Vague symptoms, such as flulike illness and rash | Oncology, targeted subspecialty |
Metabolic
TUMOR LYSIS SYNDROME
Tumor lysis syndrome is triggered by rapid, acute cell lysis caused by cancer treatment. It is often associated with chemotherapy but can also occur after radiation and biologic therapies.6 The release of intracellular products (e.g., uric acid, phosphates, calcium, potassium) overwhelms the body's homeostasis.7 With increased use of ambulatory infusion centers, family physicians may encounter tumor lysis syndrome when reviewing standard post–cancer treatment laboratory results or if a patient experiences known complications.
Although the frequency of tumor lysis syndrome is increasing in those with solid tumors, it is most common with hematologic malignancies, particularly acute leukemia and high-grade lymphomas. Tumor lysis syndrome usually presents within seven days of cancer treatment, and patients with preexisting renal insufficiency are at increased risk.7 Patients commonly present with azotemia, hyperuricemia, hyperphosphatemia, hyperkalemia, hypocalcemia, and acute renal failure, quantified by the Cairo-Bishop definitions7,8 (Table 29).

| ≥ 2 of the following in one 24-hour period within 3 days before or 7 days after the initiation of chemotherapy |
| Calcium ≤ 7.0 mg per dL (1.75 mmol per L) or 25% decrease from baseline |
| Phosphorus ≥ 4.5 mg per dL (1.45 mmol per L) in adults, 6.5 mg per dL (2.10 mmol per L) in children, or 25% increase from baseline |
| Potassium ≥ 6.0 mEq per L (6.0 mmol per L) or 25% increase from baseline |
| Uric acid ≥ 8.0 mg per dL (476 μmol per L) or 25% increase from baseline |
| Clinical tumor lysis syndrome is laboratory criteria from above plus ≥ 1 of the following |
| Cardiac arrhythmia or sudden death |
| Creatinine ≥ 1.5 times the upper limit of normal for age |
| Seizure |
Prevention and stabilization are attempted by initiating vigorous rehydration, maintaining urine output, reducing baseline uric acid levels, and limiting potassium and phosphorus intake during high-risk periods (i.e., the three days before and the seven days after initiation of cancer treatment).8,10 Prompt referral to an inpatient oncology team is recommended. In the setting of severe acute renal insufficiency, early hemodialysis can be key, necessitating nephrology referral.6
HYPERCALCEMIA OF MALIGNANCY
Hypercalcemia occurs in 10% to 30% of patients with cancer and is characterized by a serum calcium level of more than 10.5 mg per dL (2.63 mmol per L).11 It is most often associated with multiple myeloma and breast cancer, but is also common in squamous cell carcinomas of the head or neck, lung, kidney, or cervix.12 The causes of hypercalcemia fall into three general categories: humoral, bone invasion, and rare causes.11 Humoral causes, such as production of parathyroid hormone–related protein and increased vitamin D3, are most common (80% of cases). Hypercalcemia from bone invasion and local osteolysis by cytokines accounts for approximately 20% of cases. Rare causes include immobilization, medications, and parathyroid carcinoma.12,13
Symptoms of hypercalcemia include progressive decline in mental function, weakness, anorexia, thirst, constipation, nausea, vomiting, decreased urine output, and coma. Serum calcium measurements should always be adjusted for albumin levels because of possible malnourishment. Prompt stabilization includes aggressive rehydration until there is euvolemia, followed by careful diuresis with furosemide (Lasix). Whether discovered incidentally or during the course of treatment, hypercalcemia in patients with cancer should prompt urgent subspecialty referral (e.g., oncology, endocrinology, nephrology). Intravenous bisphosphonate therapy and newer monoclonal antibodies (e.g., denosumab) inhibit osteoclastic activity.14,15 Hypercalcemia of malignancy has a poor prognosis, with a median survival of 35 days from diagnosis.8
SYNDROME OF INAPPROPRIATE ANTIDIURETIC HORMONE
Syndrome of inappropriate antidiuretic hormone (SIADH) should be suspected in patients with cancer who present with hyponatremia (a sodium level of less than 135 mEq per L [135 mmol per L]). Early recognition is crucial because severe hyponatremia is associated with poor outcomes.16 Small cell lung cancer often is the ectopic source of the antidiuretic hormone production, although other tumors (head or neck) and certain chemotherapy agents (particularly vinca alkaloids, platinum compounds, and alkylating agents) can also cause SIADH.3,17
Patients with SIADH may present with gastrointestinal and neurologic symptoms. There are few physical examination findings associated with SIADH, although papilledema and pathologic reflexes (e.g., Babinski sign) are occasionally present.16 Laboratory testing often reveals hyponatremia, decreased serum osmolarity, and concentrated urine.
Although treating the cancer is primary, recent studies acknowledge that aggressive treatment of the hyponatremia itself improves patient outcomes.16,17 Fluid restriction (limit to 500 to 1,000 mL per day) is an important component of SIADH management. Slow correction of serum sodium (less than 12 mEq per L [12 mmol per L] in 24 hours and less than 18 mEq per L [18 mmol per L] in 48 hours) avoids central pontine myelinolysis; hypertonic saline is used when hyponatremia develops rapidly.17 Newer agents such as tolvaptan (Samsca), a selective vasopressin V2 receptor antagonist that increases free water excretion, are emerging, although there are concerns about liver injury.18 Regardless, patients presenting with severe symptoms are best managed with oncology and nephrology referral.19
Hematologic
FEBRILE NEUTROPENIA
Febrile neutropenia is one of the most common complications related to cancer treatment, particularly chemotherapy. The highest-risk causative agents include anthracyclines, taxanes, topoisomerase inhibitors, platinums, gemcitabines, vinorelbine (Navelbine), and alkylating agents.3 Febrile neutropenia is associated with increased morbidity and mortality rates, and decreased quality of life.20 Bacterial infections are common in patients with febrile neutropenia, but fungal sources are becoming increasingly prevalent.21
Signs of febrile neutropenia include a single axillary/oral temperature of 101.3°F (38.5°C) or higher or a sustained temperature of 100.4°F (38°C) or higher for one hour, and an absolute neutrophil count (ANC) less than 500 cells per mm3 or an expected decrease of ANC to less than 500 cells per mm3 in the next 48 hours. Patients with cancer who present with fever soon after chemotherapy should receive blood cultures and inpatient treatment with empiric antibiotics until they are afebrile for 48 to 72 hours and ANC levels are at least 500 cells per mm3 for 72 hours.22 Recent studies indicate that emergent use of antibiotics in patients with cancer who present with febrile neutropenia (optimally within 30 minutes of presentation) improves survival rates.22 Consultation with oncology and possibly infectious disease is indicated.
HYPERVISCOSITY SYNDROME
Hyperviscosity syndrome is most common in patients with Waldenström macroglobulinemia (10% to 30%), leukemia, and multiple myeloma.23 With hyperviscosity syndrome, elevated levels of circulating serum immunoglobulins coat the cells, causing increased blood viscosity, sludging of blood, and hypoperfusion.3 Signs and symptoms of hyperviscosity syndrome include spontaneous bleeding, shortness of breath, and neurologic deficits (e.g., peripheral neuropathies). “Sausage-like” hemorrhagic retinal veins are pathognomonic. A serum viscosity of more than 4 cP suggests hyperviscosity syndrome.24
Treatment includes plasmapheresis followed by targeted chemotherapy. Red blood cell and platelet transfusion should be avoided if hyperviscosity syndrome is suspected. Inpatient management is indicated to address the precipitating malignancy and includes hematology and oncology consultation.3
Structural
SUPERIOR VENA CAVA SYNDROME
Superior vena cava syndrome is caused by gradual compression of the superior vena cava where it enters the right atrium, leading to edema and retrograde flow.24 Lung cancer is the most common malignant cause, although it can also be caused by lymphoma, metastatic mediastinal tumors or lymph nodes, and indwelling catheters. 2 Facial edema is the hallmark finding of superior vena cava syndrome. Other signs and symptoms may include cough, dyspnea at rest, hoarseness, chest and shoulder pain, and swelling or discoloration of the neck or upper extremities.2 Often, collateral venous circulation causes distension of the superficial veins in the chest wall.3,24 Although superior vena cava syndrome is a clinical diagnosis (Figure 125 ), contrast-enhanced computed tomography of the chest is most commonly used to confirm clinical suspicion.3,24
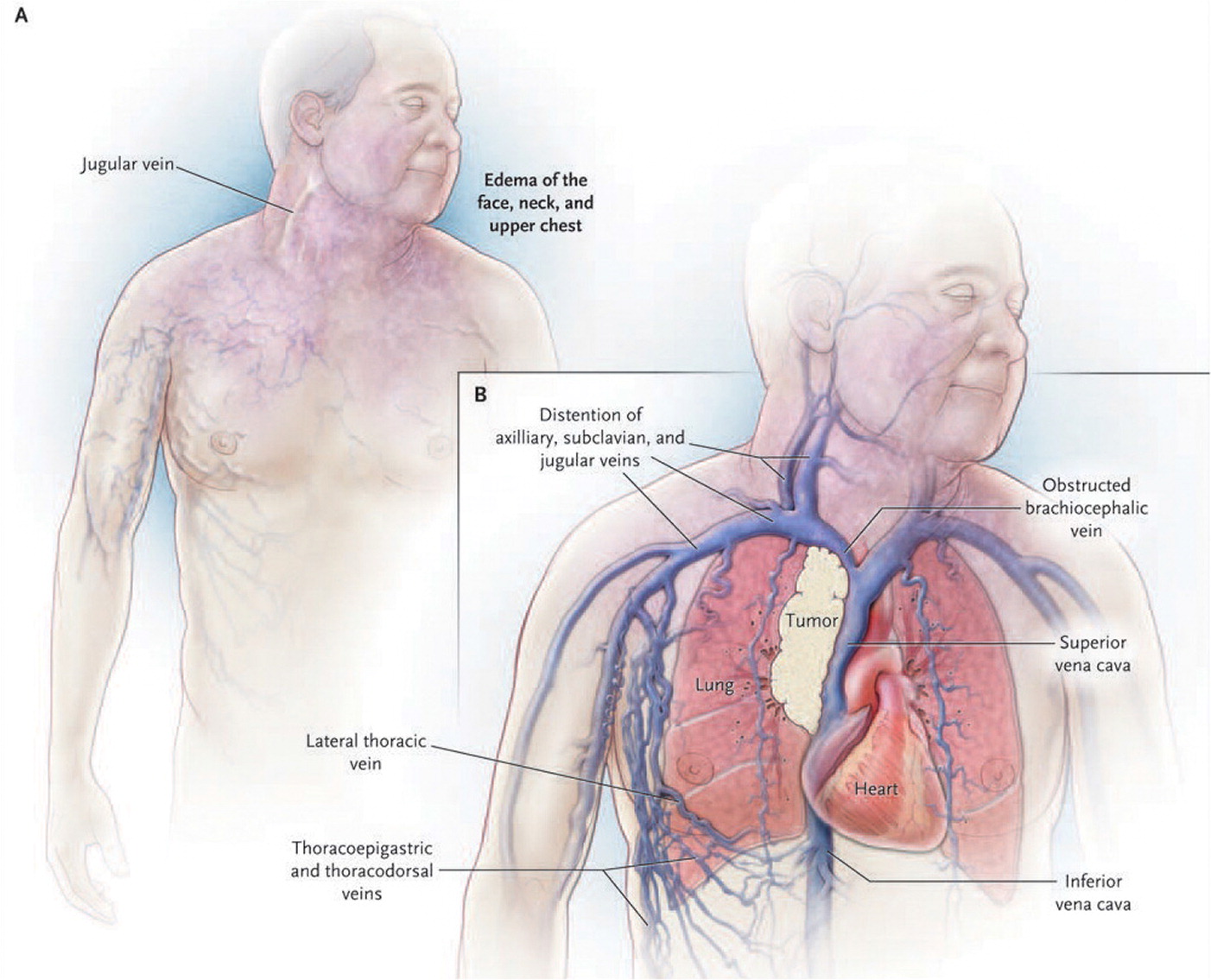
Management can include radiation, steroids, and chemotherapy to reduce a malignant obstruction. However, intravascular stenting provides symptomatic relief within one or two days and is becoming increasingly prevalent. It is sometimes the only treatment option for recurring obstructive tumors.2,24 Patients with cancer-related superior vena cava syndrome usually have advanced disease, and median survival is six months from presentation (although many survive more than two years).2 Inpatient treatment with oncology and cardiothoracic surgery consultation, as well as consideration for palliative care consultation, is recommended.24
MALIGNANT EPIDURAL SPINAL CORD COMPRESSION
Malignant epidural spinal cord compression is caused by a tumor compressing the dural sac. It is most commonly associated with breast cancer, but is also associated with multiple myeloma, lymphoma, lung cancer, and prostate cancer. It develops in about 5% of patients with cancer and can lead to paralysis if treatment is delayed by even a few hours.24 The most common presenting symptom is new-onset back pain, particularly pain that worsens when lying down, and these symptoms should be evaluated as spinal cord compression until it is ruled out.3,26 Pain with percussion of the vertebral bodies is characteristic of this condition.3 Pain of malignant epidural spinal cord compression is more commonly accompanied by motor than sensory deficits, although both can occur. Pain is often progressive; later cauda equina–type neurologic signs (incontinence and loss of sensory function) are associated with permanent paraplegia.24 Plain radiography can show lesions in patients with solid tumors, but magnetic resonance imaging is recommended.3 A magnetic resonance imaging scan showing spinal cord compression is available at http://www.bimjonline.com/Imageoftheweek/Image28.05.2012/Figure%201.jpg.
Whether or not neurologic symptoms are present, malignant epidural spinal cord compression warrants a multidisciplinary approach, including neurosurgery consultation. Steroid treatment and/or surgery can preserve motor and sensory function. Radiation therapy is increasingly being used as adjunctive treatment.26–28
MALIGNANT PERICARDIAL EFFUSIONS
Malignant pericardial effusions occur in 20% to 34% of patients with cancer. The overall prognosis is poor, with a median survival time of 130 to 140 days.2,24,29,30 Associated cancers include lung, esophageal, and breast cancers; lymphoma; leukemia; and melanoma.1,24,31 Radiation therapy, multiple chemotherapy agents, infection, and autoimmune reactions are other sources.29,31 Rapidly accumulating effusions cause symptoms with as little as 200 mL of fluid, whereas those accumulating more slowly can contain up to 2 L of fluid before severe symptoms are noted.2,24 Most often, patients present with dyspnea, chest pain, or palpitations. Pulsus paradoxus (see video at https://m.youtube.com/watch?feature=youtu.be&v=jTsjCZ9QxW8) occurs in 77% of tamponade cases and 30% of malignant pericardial effusions.24 The Beck triad (muffled heart sounds, hypotension, increased jugular venous pressure) is observed in rapidly accumulating effusions, but is less common in slowly developing eff usions.2,24 Electrocardiographic findings include low-amplitude wave forms and electrical alternans.24 Although chest radiography will show an enlarged and widened cardiac silhouette, echocardiography is the preferred diagnostic study.2,24
Acute symptoms are relieved with pericardiocentesis alone. However, recent studies suggest improved outcomes with more permanent solutions, such as pericardial windows or pericardiocentesis with indwelling pericardial catheter drainage, and chemotherapy when indicated.24,29–31 Consultation with oncology, cardiothoracic surgery, and palliative care are recommended.
Treatment Adverse Effects
EXTRAVASATION INJURIES SECONDARY TO CHEMOTHERAPY
Extravasation refers to a liquid accidentally leaking into surrounding tissues rather than staying in the blood vessel as intended. With toxic chemotherapies, various extravasation injuries are possible.32 Prevention is key and can help avoid the need for debridement, skin grafting, or even amputation.33 Pain, erythema, and swelling that progress to blanching, blistering, discoloration, and necrosis of the skin can occur.33 Prompt diagnosis is achieved by team training and vigilance, including patient education.34 Early treatment is crucial and includes stopping the infusion immediately and leaving the cannula in place while the next steps are determined.32 In addition to consulting oncology, consultation with dermatology and plastic surgery may be needed.
GASTROINTESTINAL PROBLEMS
Approximately 17% of acute care sought by patients with cancer is attributable to gastrointestinal problems, which are increasingly associated with opioid or immunologic therapy. Abdominal pain, nausea, vomiting, diarrhea, constipation, and dehydration are common in those undergoing cancer treatments.35 In these acute scenarios, stabilization includes fluid resuscitation, and use of antiemetics and antidiarrheals followed by further investigation, such as testing for Clostridium difficile infection, cytomegalovirus infection, and reactivation of latent hepatitis. Obstruction can be benign or malignant. With gastrointestinal bleeding, taking a careful history is key—patients who have received pelvic radiation therapy are at increased risk of bowel ischemia and perforation. Lactose intolerance, small bowel bacterial overgrowth, and bile acid malabsorption often develop after chemotherapy.36
The use of probiotics containing Lactobacillus is one new recommended preventive measure for diarrhea induced by chemotherapy or radiation therapy.37 Consultation with oncology, gastroenterology, general surgery, and infectious disease can be helpful depending on the presenting gastrointestinal problem and imaging findings.
COMPLICATIONS OF RADIATION TREATMENT
Radiation-related dermatitis, cardiovascular disease, esophagitis, cystitis, sexual dysfunction, and depression are common.38 Adverse reactions can occur at varying times posttreatment, ranging from those that resolve within a few months to new cancers occuring a decade or more after treatment. Complications are influenced by type of cancer, type of radiation, age at diagnosis, and total dose of radiation received.38 Various subspecialty consultations are necessary when managing these patients. A family physician may be asked if radiation treatment can harm family members; guidelines for patient contacts are outlined in eTable A. Oncology consultation helps each patient's care team determine whether to limit contact and for how long.
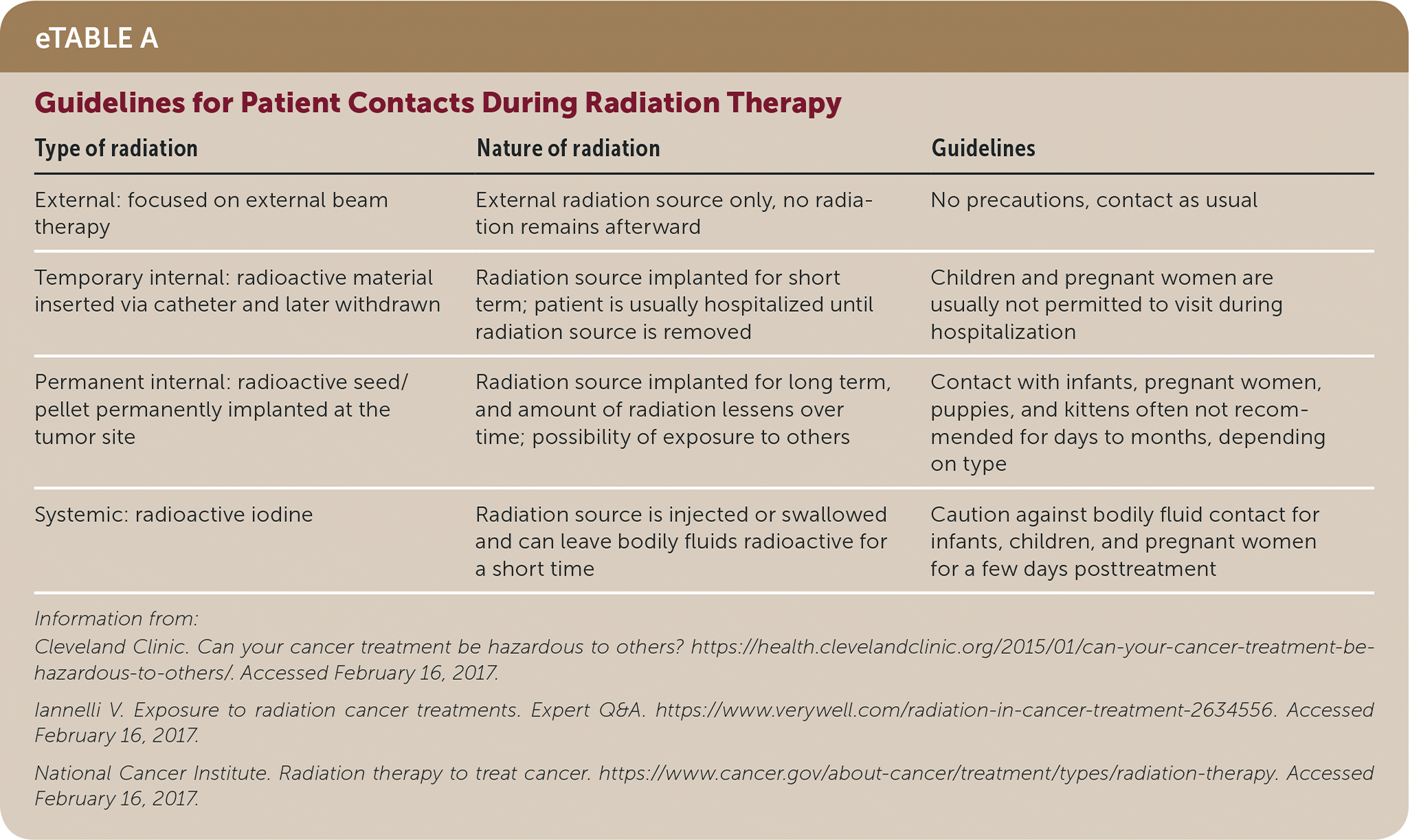
| Type of radiation | Nature of radiation | Guidelines |
|---|---|---|
| External: focused on external beam therapy | External radiation source only, no radiation remains afterward | No precautions, contact as usual |
| Temporary internal: radioactive material inserted via catheter and later withdrawn | Radiation source implanted for short term; patient is usually hospitalized until radiation source is removed | Children and pregnant women are usually not permitted to visit during hospitalization |
| Permanent internal: radioactive seed/pellet permanently implanted at the tumor site | Radiation source implanted for long term, and amount of radiation lessens over time; possibility of exposure to others | Contact with infants, pregnant women, puppies, and kittens often not recommended for days to months, depending on type |
| Systemic: radioactive iodine | Radiation source is injected or swallowed and can leave bodily fluids radioactive for a short time | Caution against bodily fluid contact for infants, children, and pregnant women for a few days posttreatment |
COMPLICATIONS OF IMMUNOTHERAPY
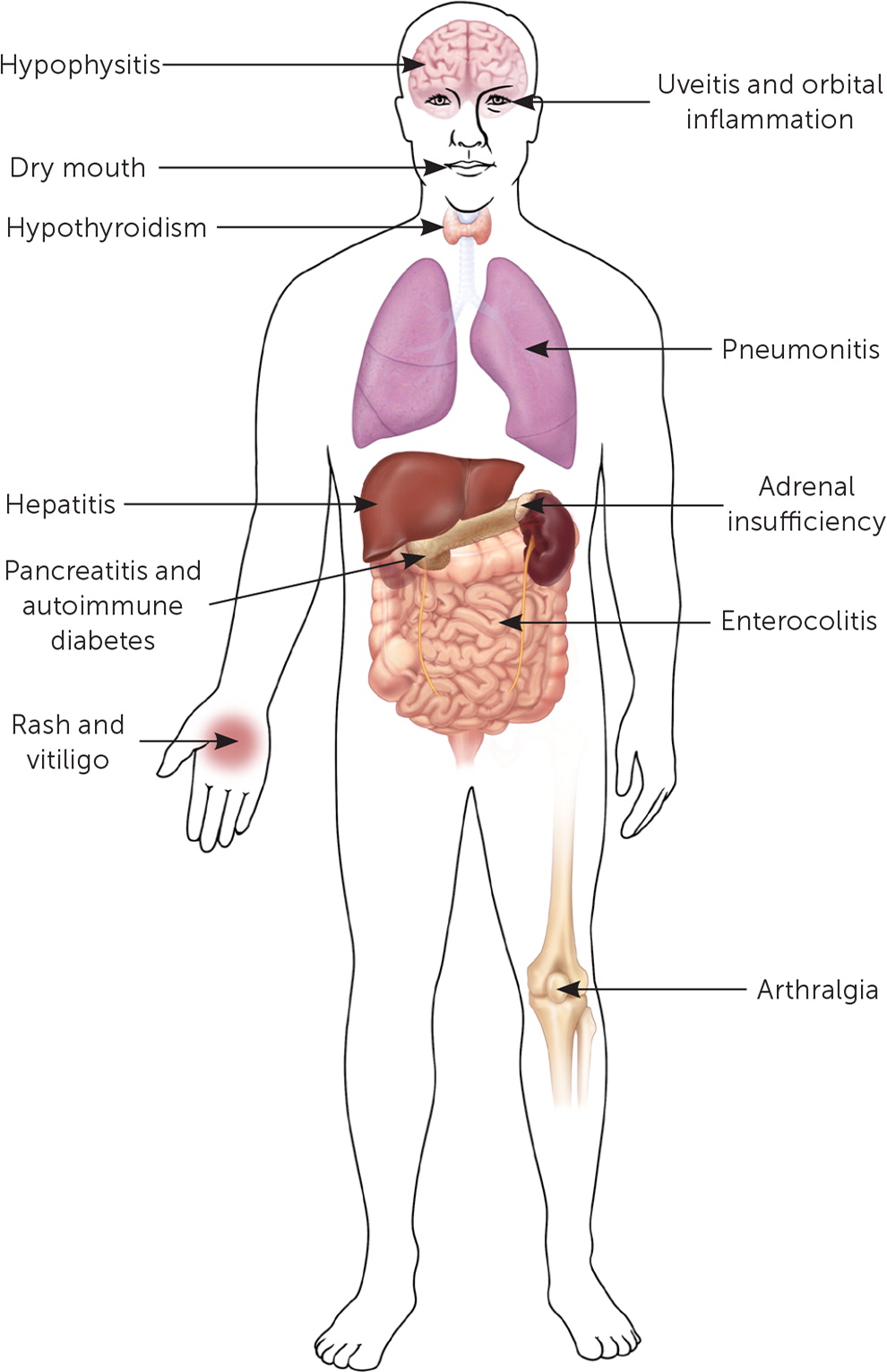
With rapid developments in cancer research, new immunologic agents are becoming increasingly available. Depending on the treatment modality used (cytokines, vaccines, adoptive cell therapy, or checkpoint inhibitors), multiple toxic expressions can occur with cancer immunotherapy and are collectively referred to as immune-related adverse effects39–41 (Figure 2). The clinical spectrum of these events spans from a nonspecific and vague flulike illness and rash to potentially life-threatening pneumonitis and pancreatitis.40–42 High level of suspicion with prompt oncology and targeted subspecialty consultation is necessary.
This article updates a previous article on this topic by Higdon and Higdon.5
Data Sources: A PubMed/MEDLINE search was completed using the key words neoplasms, tumor lysis syndrome, hypercalcemia of malignancy, syndrome of inappropriate antidiuretic hormone, febrile neutropenia, hyperviscosity syndrome, superior vena cava syndrome, epidural spinal cord compression, malignant pericardial effusion, emergency, drug therapy, therapy, chemotherapy, radiotherapy, surgery, diet therapy, biologic therapy, and immunologic therapy. The search was limited to the previous five years and included meta-analyses, randomized controlled trials, clinical trials, case studies, and reviews. Essential Evidence Plus and Google Scholar were also searched. Search dates: February 3, 6, and 16, 2017.
