
Am Fam Physician. 2019;99(6):392-394
Author disclosure: No relevant financial affiliations.
Lofexidine (Lucemyra) is an alpha-adrenergic agonist and a structural analogue of clonidine. Lofexidine is labeled for use in the mitigation of withdrawal symptoms for up to 14 days during abrupt discontinuation of opioids in adults.1
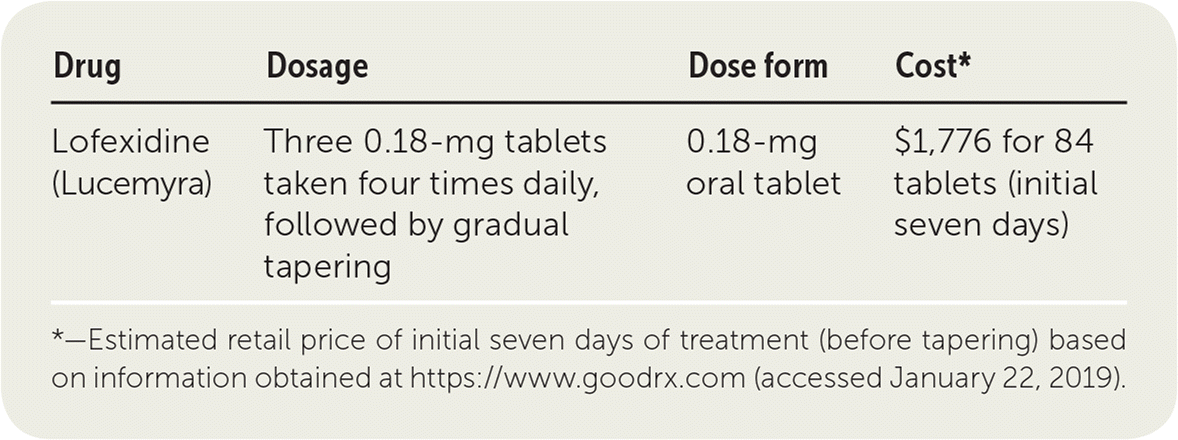
| Drug | Dosage | Dose form | Cost* |
|---|---|---|---|
| Lofexidine (Lucemyra) | Three 0.18-mg tablets taken four times daily, followed by gradual tapering | 0.18-mg oral tablet | $1,776 for 84 tablets (initial seven days) |
Safety
Serious adverse effects of lofexidine are relatively rare and have been shown to be dose related. No adverse effects were reported by a group of 229 patients treated with 2.4 mg of lofexidine per day.2 At the higher dosage of 3.2 mg per day, serious adverse effects included suicidal ideation (0.5%), bradycardia (0.8%), and hypotension or syncope (1.1%). Lofexidine should not be prescribed for children or adolescents, pregnant or nursing women, or for patients older than 65 because it has not been studied in these groups. It should not be used in patients with severe coronary insufficiency, recent myocardial infarction, stroke, renal failure, or significant bradycardia. Central nervous system depression is a serious risk, especially if lofexidine is taken in combination with benzodiazepines, alcohol, barbiturates, or other sedating drugs. Lofexidine should only be prescribed within a program of close medical supervision. Maximal doses of lofexidine may prolong the QT interval by an additional 10 milliseconds. This should be closely monitored in high-risk patients, such as those with a baseline QT interval greater than 450 milliseconds, or those with concomitant methadone use.1 Lofexidine is not an opioid and does not affect psychological cravings. Patients should transition to a long-term treatment plan for opioid use disorder.
Tolerability
Lofexidine is a centrally-acting sympatholytic and has been shown to cause dose-related hypotension averaging about 10 mm Hg, although this is typically not clinically significant. At a daily dosage of 12 tablets (2.16 mg), patients may experience dry mouth (10%), sedation (13%), dizziness (19%), bradycardia (24%), and hypotension (30%).1 Abrupt discontinuation of lofexidine can cause rebound hypertension, as well as diarrhea, chills, and hyperhidrosis. It is recommended that patients discontinue use gradually.
Effectiveness
Effectiveness of lofexidine can be measured by severity of withdrawal symptoms, duration of the episode, and likelihood of completing treatment. Two major treatment strategies exist; gradual discontinuation using a prescription opioid or abrupt discontinuation without using an opioid.
Both lofexidine and clonidine are more effective than placebo for abrupt discontinuation, based on a Cochrane review of moderate-quality clinical trials.3 Three double-blind, randomized controlled trials that included 158 patients and compared lofexidine with clonidine showed equal effectiveness, although more patients treated with clonidine experienced hypotension or feeling weak or tired.3
In the broader context of withdrawal treatment, lofexidine or clonidine may replace or complement the two major opioids used for this indication: methadone and buprenorphine. These opioids are tightly regulated and require special training and certifications for their prescription. Neither lofexidine nor clonidine is as effective as management with buprenorphine, which controls symptoms better and leads to a higher likelihood of completing withdrawal treatment (number needed to treat = 4; 95% confidence interval, 3 to 6).4 Alpha-adrenergic agonists were previously found to be as effective as methadone but with more adverse effects and a shorter duration of withdrawal.5
Because alpha-adrenergic agonists are not opioids, they are less effective at treating psychological cravings and are not appropriate for long-term management of opioid use disorder. However, as an adjunct to an outpatient buprenorphine maintenance program, clonidine was effective in prolonging the duration of abstinence, which suggests it may play a useful role in this setting.6
Price
Following recommended dosing, 84 tablets may be required per withdrawal episode. The cost of lofexidine for the first seven days (84 tablets) is $1,776. This is considerably higher than its almost identical analogue, clonidine, which costs about $9 for a 30-day supply.
Simplicity
The starting dosage of lofexidine is typically three 0.18-mg tablets taken four times per day during the first five to seven days, allowing at least five to six hours between doses. Patients should not exceed four tablets per dose and should not take more than 16 tablets per day. Dosing adjustments for patients with hepatic and renal impairment are necessary and based on the level of impairment.1 Lofexidine should be discontinued gradually by decreasing one tablet per dose every one to two days.
Bottom Line
Lofexidine has been shown to be as effective as clonidine at controlling some of the symptoms of withdrawal from opioid use. It may have a small additional benefit for maintaining abstinence in patients switched to buprenorphine or methadone for long-term maintenance. However, lofexidine is expensive and has significant adverse effects, which should limit its use to patients who require symptom control not achieved by other treatments.
The views expressed are those of the author and do not reflect the official policy or position of the U.S. Air Force, the Department of Defense, or the U.S. government.
