
Am Fam Physician. 2019;100(4):209-210
Author disclosure: No relevant financial affiliations.

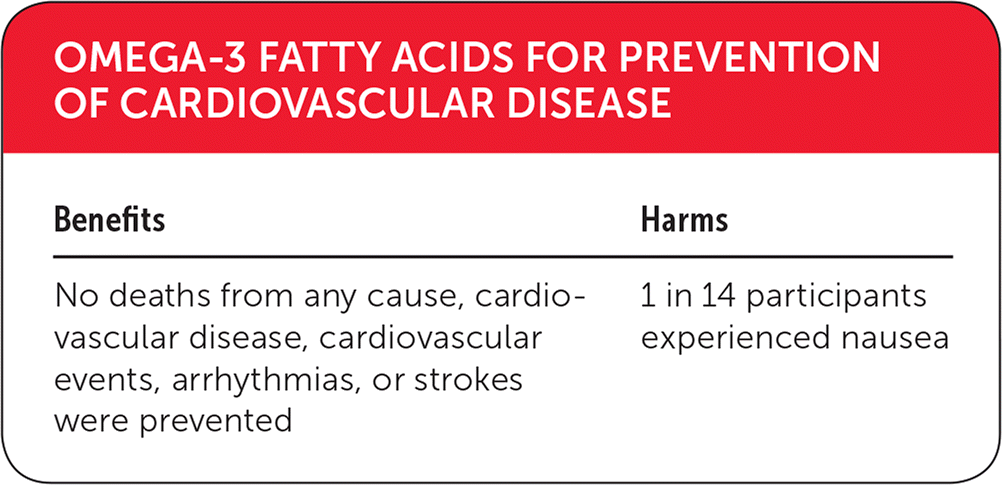
| Benefits | Harms |
|---|---|
| No deaths from any cause, cardiovascular disease, cardiovascular events, arrhythmias, or strokes were prevented | 1 in 14 participants experienced nausea |
Details for This Review
Study Population: Adults 18 years and older with varying levels of cardiovascular risk
Efficacy End Points: All-cause mortality, cardiovascular mortality, cardiovascular events, arrhythmia, stroke
Harm End Points: Nausea, abdominal pain or discomfort, diarrhea, reflux, any gastrointestinal side effect, headache or worsening migraine, joint and muscle pain, skin problems, gastrointestinal bleeding, hospitalization, pulmonary embolism (PE) or deep venous thrombosis (DVT)
Narrative: Noncommunicable diseases have overtaken communicable diseases as the major disease burden worldwide, with circulatory and cardiovascular diseases (CVD) remaining the leading cause of death globally.1 Researchers have long investigated the health effects of omega-3 polyunsaturated fatty acids (PUFAs), including eicosapentaenoic acid (EPA; C20:5) and docosahexaenoic acid (DHA; C22:6), on prevention of CVD. Dietary fatty acids may be oxidized for energy, stored in adipose tissue, or further metabolized to various long-chain PUFAs. Membrane-derived PUFAs serve as substrates for formation of eicosanoid effectors (omega-3 and omega-6).2 The effectors derived from omega-3 PUFAs are less inflammatory and platelet aggregating than their omega-6-derived counterparts.2
The primary source of nonprescription omega-3 PUFA supplements is fish oil.3 In 2012, 7.8% of U.S. adults (18.8 million) reported consuming a fish oil dietary supplement within the past 30 days.4 An American Heart Association report suggested that omega-3 PUFA supplements may reduce death from coronary heart disease (CHD), possibly through a reduction in ischemia-induced sudden cardiac death, among patients with previous CHD. The report found that these supplements do not reduce the incidence of recurrent nonfatal myocardial infarction.3 Because benefits likely outweigh the risks, the American Heart Association report offered a Class IIa recommendation (benefits outweigh risks; additional studies with focused objectives needed; it is reasonable to administer treatment) for the use of omega-3 PUFA supplements for the secondary prevention of CHD death.3
The meta-analysis discussed here analyzes the effectiveness of dietary omega-3 PUFA supplementation with EPA (fish-derived; C20:5), DHA (fish-derived; C22:6) and alpha-linolenic acid (plant-derived; C18:3) to improve all-cause mortality, cardiovascular deaths, cardiovascular events, arrhythmias, and stroke. The meta-analysis included 79 randomized controlled trials (RCTs), including 112,059 participants, published before April 2017.5 The meta-analysis excluded 27 trials that were ongoing; 33 trials included patients with previous cardiovascular risk (secondary prevention); and 46 trials included patients with no previous cardiovascular risk (primary prevention). The follow-up period varied from 12 to 72 months. Omega-3 PUFA supplementation was performed via capsule or medicinal oils, omega-3 PUFA-enriched foods, or via dietary advice to increase omega-3 PUFA intake. Because of the small number of trials assessing long-chain omega-3 fatty acids, EPA and DHA were analyzed in aggregate.5
BENEFITS
Long-Chain Omega-3 Polyunsaturated Fatty Acids. No significant benefits were noted with long-chain omega-3 PUFA supplementation (EPA, DHA) for preventing all-cause mortality (39 RCTs; 92,653 participants; 4,544 CVD deaths), cardiovascular mortality (25 RCTs; 67,722 participants), cardiovascular events (38 RCTs; 90,378 participants), arrhythmia (28 RCTs; 53,796 participants), or stroke (28 RCTs; 89,358 participants).
Alpha-Linolenic Acid. No significant benefits were noted with alpha-linolenic acid supplementation for preventing all-cause mortality (five RCTs; 19,327 participants; 459 deaths), cardiovascular mortality (four RCTs; 18,619 participants; 219 CVD deaths), cardiovascular events (five RCTs; 19,327 participants; 884 CVD events), arrhythmia (one RCT; 4,837 participants; 141 events), or stroke (five RCTs; 19,327 participants; 884 CVD events).5
HARMS
The meta-analysis assessed nonserious side effects (nausea, abdominal pain or discomfort, diarrhea, reflux, any gastrointestinal side effect, headache or worsening migraine, joint lumbar and muscle pain, or skin problems) and serious adverse effects (gastrointestinal hospitalization, bleeding, PE or DVT).5
Long-Chain Omega-3 Polyunsaturated Fatty Acids. There was no suggestion that long-chain omega-3 significantly increased nonserious side effects (aggregate) with high heterogeneity.5 Nausea was increased in the long-chain omega-3 PUFA group (relative risk = 1.76; 95% CI, 1.25 to 2.48; I2 = 0%; number needed to treat = 14; five RCTs; 1,234 participants). All other nonserious side effects were not statistically different.5 Serious adverse events were not increased with long-chain omega-3 intake.
Alpha-Linolenic Acid. Supplementation did not increase side effect–mediated study withdrawal.5 Insufficient data were available to assess other nonserious side effects. Data were also insufficient for assessing the risk of PE or DVT (one RCT; 708 participants; one event). No data were available for any other serious adverse events.
Caveats: Omega-3 PUFA-enriched foods (e.g., dietary fish) may have different health effects than capsule or medicinal oil omega-3 PUFA supplements, because they may replace consumption of less healthy foods (leading to reduced saturated fat intake) and provide other additional nutrients (e.g., protein, selenium, iodine, calcium, magnesium). The subgroup analysis performed in the meta-analysis was underpowered to detect a statistically significant difference between the dietary advice subgroups (enriched food vs. capsule or medicinal oil). Daily omega-3 PUFA intake in such studies is not quantifiable because dietary practices vary across patients. It remains unclear whether studies using this design skewed the potential effects of studies using a more traditional design. Furthermore, the assessment of EPA and DHA in aggregate potentially masked the benefit of any singular agent.
The follow-up duration of at least 12 months may not have been long enough to detect any significant impact on mortality and cardiovascular outcomes.
Evidence from funnel-plots reported in the meta-analysis suggest some small study bias, indicating some smaller studies showing increased risk of CVD outcomes with omega-3 PUFA supplement use may be missing. Additionally, the data are limited by significant heterogeneity. This likely reflects the variable means by which data points were collected across trials.
Lastly, this analysis did not collate data on cancers and neurologic problems associated with polychlorinated biphenyls or mercury in fish oils.5
In summary, high-quality evidence suggests that long-chain omega-3 PUFAs do not prevent mortality (all-cause or cardiovascular) or CVD events when used as primary or secondary prevention. Because of a lack of any benefit but relative safety, we have assigned a color recommendation of red (no benefits) to this treatment.
Copyright © 2019 MD Aware, LLC (theNNT.com). Used with permission.
This series is coordinated by Dean A. Seehusen, MD, MPH, AFP Assistant Medical Editor, and Daniel Runde, MD, from the NNT Group.
A collection of Medicine by the Numbers published in AFP is available at https://www.aafp.org/afp/mbtn.
