
Am Fam Physician. 2020;101(3):179-181
Author disclosure: No relevant financial affiliations.
Key Clinical Issue
What are the adverse events of antidepressants prescribed to treat major depressive disorder in adults 65 years and older?
Evidence-Based Answer
Selective serotonin reuptake inhibitors (SSRIs) cause adverse events at a similar frequency to placebo and have lower discontinuation rates than tricyclic antidepressants during up to 12 weeks of treatment. (Strength of Recommendation [SOR]: B, based on inconsistent or limited-quality patient-oriented evidence.) Serotonin-norepinephrine reuptake inhibitors (SNRIs) cause more adverse events and greater discontinuation of therapy during up to 12 weeks of treatment compared with placebo. (SOR: B, based on inconsistent or limited-quality patient-oriented evidence.) Duloxetine increases the risk of falls over 12 to 24 weeks of treatment compared with placebo.1 (SOR: B, based on inconsistent or limited-quality patient-oriented evidence.)
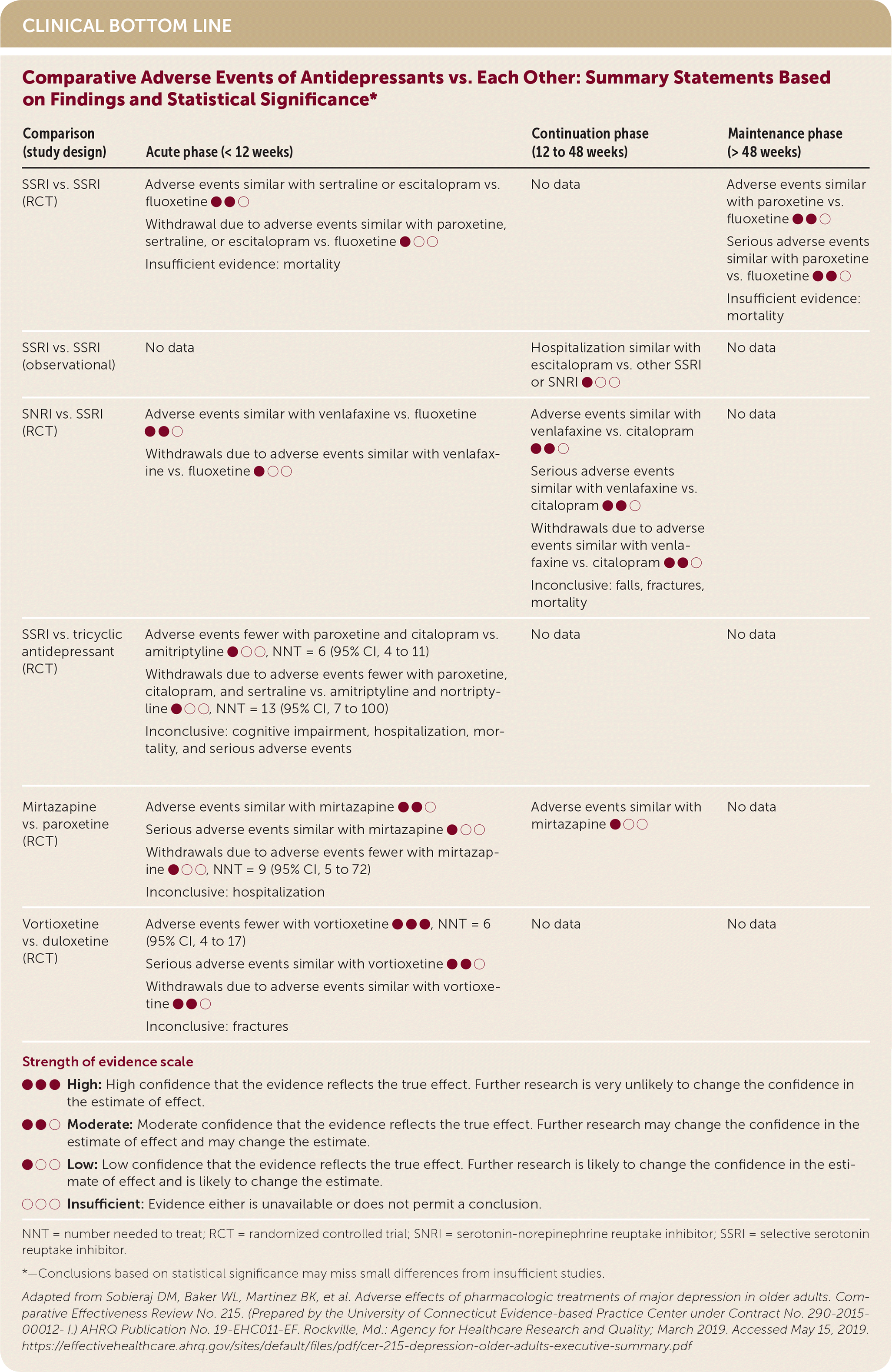
| Comparison (study design) | Acute phase (< 12 weeks) | Continuation phase (12 to 48 weeks) | Maintenance phase (> 48 weeks) |
|---|---|---|---|
| SSRI vs. SSRI (RCT) | Adverse events similar with sertraline or escitalopram vs. fluoxetine ●●○ Withdrawal due to adverse events similar with paroxetine, sertraline, or escitalopram vs. fluoxetine ●○○ Insufficient evidence: mortality | No data | Adverse events similar with paroxetine vs. fluoxetine ●●○ Serious adverse events similar with paroxetine vs. fluoxetine ●●○ Insufficient evidence: mortality |
| SSRI vs. SSRI (observational) | No data | Hospitalization similar with escitalopram vs. other SSRI or SNRI ●○○ | No data |
| SNRI vs. SSRI (RCT) | Adverse events similar with venlafaxine vs. fluoxetine ●●○ Withdrawals due to adverse events similar with venlafaxine vs. fluoxetine ●○○ | Adverse events similar with venlafaxine vs. citalopram ●●○ Serious adverse events similar with venlafaxine vs. citalopram ●●○ Withdrawals due to adverse events similar with venlafaxine vs. citalopram ●●○ Inconclusive: falls, fractures, mortality | No data |
| SSRI vs. tricyclic antidepressant (RCT) | Adverse events fewer with paroxetine and citalopram vs. amitriptyline ●○○, NNT = 6 (95% CI, 4 to 11) Withdrawals due to adverse events fewer with paroxetine, citalopram, and sertraline vs. amitriptyline and nortriptyline ●○○, NNT = 13 (95% CI, 7 to 100) Inconclusive: cognitive impairment, hospitalization, mortality, and serious adverse events | No data | No data |
| Mirtazapine vs. paroxetine (RCT) | Adverse events similar with mirtazapine ●●○ Serious adverse events similar with mirtazapine ●○○ Withdrawals due to adverse events fewer with mirtazapine ●○○, NNT = 9 (95% CI, 5 to 72) Inconclusive: hospitalization | Adverse events similar with mirtazapine ●○○ | No data |
| Vortioxetine vs. duloxetine (RCT) | Adverse events fewer with vortioxetine ●●●, NNT = 6 (95% CI, 4 to 17) Serious adverse events similar with vortioxetine ●●○ Withdrawals due to adverse events similar with vortioxetine ●●○ Inconclusive: fractures | No data | No data |
Practice Pointers
Approximately 15% to 20% of adults 65 years and older have depression.2 The American Psychiatric Association recommends antidepressants as an initial treatment option. SSRIs, SNRIs, mirtazapine, and bupropion are suggested as first-line agents for the general adult population and for older adults.3 Meta-analyses of randomized controlled trials (RCTs) have shown that among adults 60 years and older, antidepressants are more effective than placebo for treating depression, although effect size is modest.4 However, the 2019 Beers Criteria for Potentially Inappropriate Medication Use in Older Adults recommend that SSRIs, SNRIs, and tricyclic antidepressants be avoided in older adults with a history of falls or fractures.5 The Agency for Healthcare Research and Quality (AHRQ) review aims to synthesize research on adverse event profiles of antidepressants in older adults.
This AHRQ review includes 19 RCTs and two observational studies. The RCTs include only patients 65 years and older, mostly with moderate severity major depressive disorder. Trials studied the acute phase of major depressive disorder (less than 12 weeks), the continuation phase (12 to 48 weeks), or the maintenance phase (more than 48 weeks). Although the authors attempted to evaluate SSRIs and SNRIs by drug class, most studies included only a few individual drugs.
In trials comparing SSRIs (paroxetine, citalopram, or sertraline) head-to-head with tricyclic antidepressants (amitriptyline or nortriptyline) during the acute phase of treatment, patients taking SSRIs were less likely to withdraw from trials because of adverse events. One large cohort study of people 65 years and older who had depression found that SSRIs were associated with an increased risk of falls, fractures, and all-cause mortality compared with no antidepressant use over a longer treatment period (median of 364 days).6
One RCT of the SNRI duloxetine reported an increased risk of treatment withdrawal due to adverse events and increased risk of falls compared with placebo over 12 to 24 weeks. Venlafaxine was associated with an increased risk of falls, fractures, and mortality compared with no antidepressant use in a large cohort study with a median treatment period of 364 days.7
There were limitations to this analysis, including significant risk of bias in seven out of 19 studies, lower than usual medication dosages in many studies, and inadequate power to detect differences in adverse events in individual RCTs.
For patients 65 years and older with major depressive disorder, first-line antidepressants are SSRIs, SNRIs, mirtazapine, and bupropion. Available evidence suggests that SNRIs have higher rates of adverse events and treatment withdrawal due to adverse events compared with placebo, and that duloxetine is associated with an increased risk of falls. Long-term comparative studies that are specifically designed to assess adverse events among adults 65 and older are needed to better inform decision-making for this population.
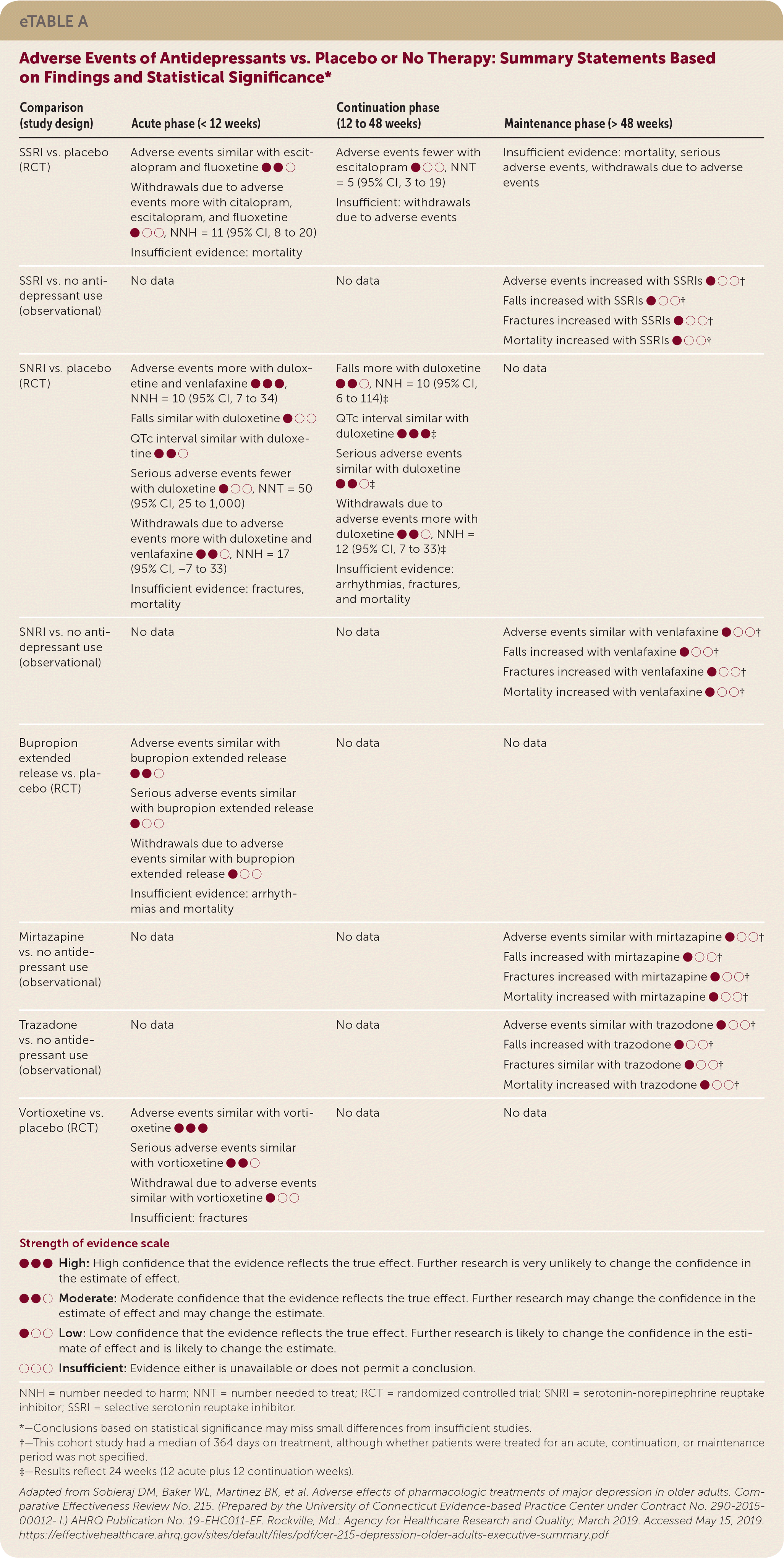
| Comparison (study design) | Acute phase (< 12 weeks) | Continuation phase (12 to 48 weeks) | Maintenance phase (> 48 weeks) |
|---|---|---|---|
| SSRI vs. placebo (RCT) | Adverse events similar with escitalopram and fluoxetine ●●○ Withdrawals due to adverse events more with citalopram, escitalopram, and fluoxetine ●○○, NNH = 11 (95% CI, 8 to 20) Insufficient evidence: mortality | Adverse events fewer with escitalopram ●○○, NNT = 5 (95% CI, 3 to 19) Insufficient: withdrawals due to adverse events | Insufficient evidence: mortality, serious adverse events, withdrawals due to adverse events |
| SSRI vs. no antidepressant use (observational) | No data | No data | Adverse events increased with SSRIs ●○○† Falls increased with SSRIs ●○○† Fractures increased with SSRIs ●○○ † Mortality increased with SSRIs ●○○ † |
| SNRI vs. placebo (RCT) | Adverse events more with duloxetine and venlafaxine ●●●, NNH = 10 (95% CI, 7 to 34) Falls similar with duloxetine ●○○ QTc interval similar with duloxetine ●●○ Serious adverse events fewer with duloxetine ●○○, NNT = 50 (95% CI, 25 to 1,000) Withdrawals due to adverse events more with duloxetine and venlafaxine ●●○, NNH = 17 (95% CI, −7 to 33) Insufficient evidence: fractures, mortality | Falls more with duloxetine ●●○, NNH = 10 (95% CI, 6 to 114)‡ QTc interval similar with duloxetine ●●●‡ Serious adverse events similar with duloxetine ●●○‡ Withdrawals due to adverse events more with duloxetine ●●○, NNH = 12 (95% CI, 7 to 33)‡ Insufficient evidence: arrhythmias, fractures, and mortality | No data |
| SNRI vs. no antidepressant use (observational) | No data | No data | Adverse events similar with venlafaxine ●○○† Falls increased with venlafaxine ●○○† Fractures increased with venlafaxine ●○○† Mortality increased with venlafaxine ●○○† |
| Bupropion extended release vs. placebo (RCT) | Adverse events similar with bupropion extended release ●●○ Serious adverse events similar with bupropion extended release ●○○ Withdrawals due to adverse events similar with bupropion extended release ●○○ Insufficient evidence: arrhythmias and mortality | No data | No data |
| Mirtazapine vs. no antidepressant use (observational) | No data | No data | Adverse events similar with mirtazapine ●○○† Falls increased with mirtazapine ●○○† Fractures increased with mirtazapine ●○○† Mortality increased with mirtazapine ●○○† |
| Trazadone vs. no antidepressant use (observational) | No data | No data | Adverse events similar with trazodone ●○○† Falls increased with trazodone ●○○† Fractures similar with trazodone ●○○† Mortality increased with trazodone ●○○† |
| Vortioxetine vs. placebo (RCT) | Adverse events similar with vortioxetine ●●● Serious adverse events similar with vortioxetine ●●○ Withdrawal due to adverse events similar with vortioxetine ●○○ Insufficient: fractures | No data | No data |
Editor's Note: American Family Physician SOR ratings are different from the AHRQ Strength of Evidence ratings. Dr. Salisbury-Afshar is an AFP contributing editor.
