
Am Fam Physician. 2020;101(6):online
Related Editorial: Integrating Breast Cancer Risk Management into Primary Care
Related Putting Prevention into Practice: Medication Use to Reduce Risk of Breast Cancer
As published by the USPSTF.
Summary of Recommendation and Evidence
The USPSTF recommends that clinicians offer to prescribe risk-reducing medications, such as tamoxifen, raloxifene, or aromatase inhibitors, to women who are at increased risk for breast cancer and at low risk for adverse medication effects (Table 1). (B recommendation).
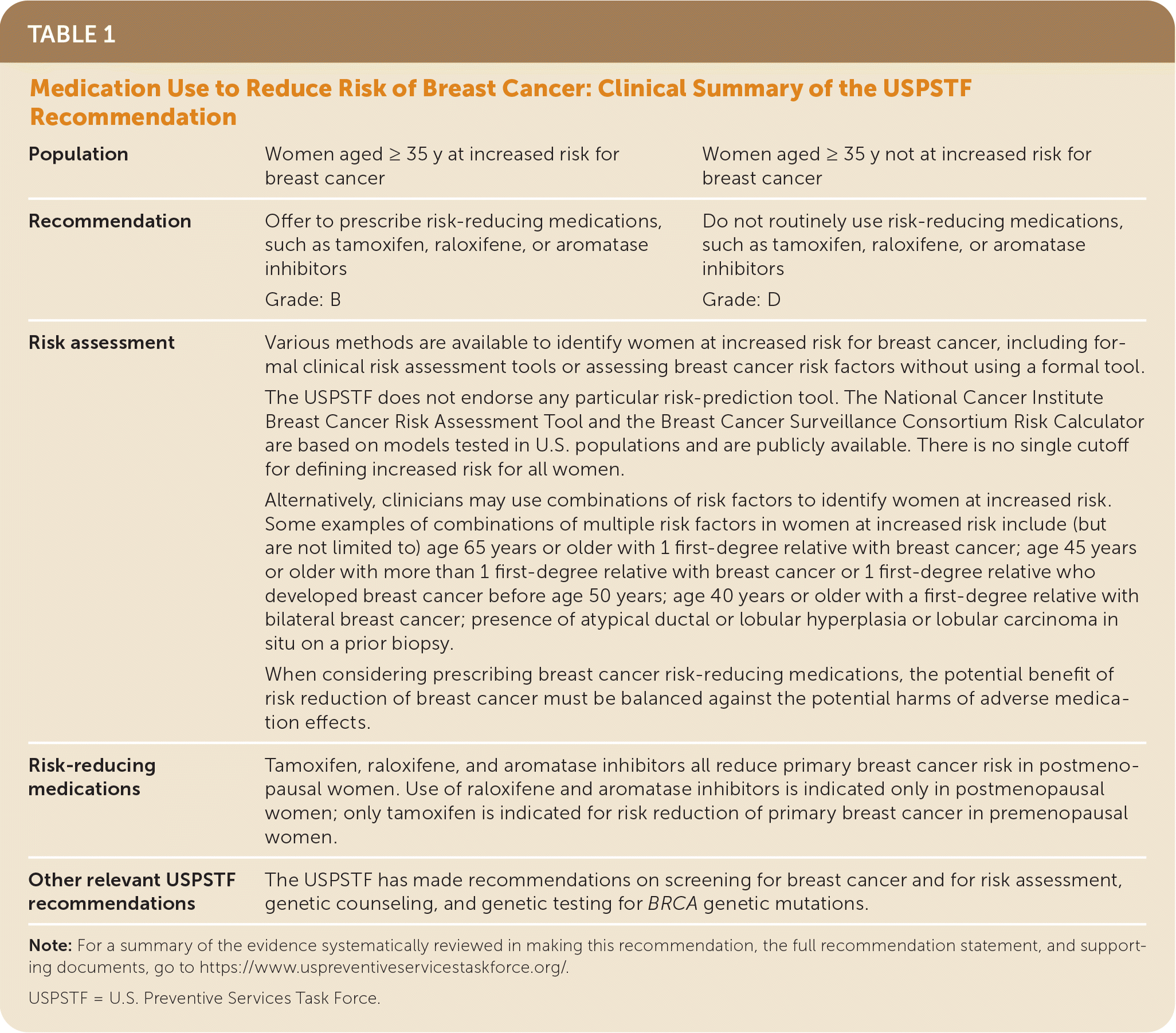
| Population | Women aged ≥ 35 y at increased risk for breast cancer | Women aged ≥ 35 y not at increased risk for breast cancer |
| Recommendation | Offer to prescribe risk-reducing medications, such as tamoxifen, raloxifene, or aromatase inhibitors Grade: B | Do not routinely use risk-reducing medications, such as tamoxifen, raloxifene, or aromatase inhibitors Grade: D |
| Risk assessment | Various methods are available to identify women at increased risk for breast cancer, including formal clinical risk assessment tools or assessing breast cancer risk factors without using a formal tool. The USPSTF does not endorse any particular risk-prediction tool. The National Cancer Institute Breast Cancer Risk Assessment Tool and the Breast Cancer Surveillance Consortium Risk Calculator are based on models tested in U.S. populations and are publicly available. There is no single cutoff for defining increased risk for all women. Alternatively, clinicians may use combinations of risk factors to identify women at increased risk. Some examples of combinations of multiple risk factors in women at increased risk include (but are not limited to) age 65 years or older with 1 first-degree relative with breast cancer; age 45 years or older with more than 1 first-degree relative with breast cancer or 1 first-degree relative who developed breast cancer before age 50 years; age 40 years or older with a first-degree relative with bilateral breast cancer; presence of atypical ductal or lobular hyperplasia or lobular carcinoma in situ on a prior biopsy. When considering prescribing breast cancer risk-reducing medications, the potential benefit of risk reduction of breast cancer must be balanced against the potential harms of adverse medication effects. | |
| Risk-reducing medications | Tamoxifen, raloxifene, and aromatase inhibitors all reduce primary breast cancer risk in postmenopausal women. Use of raloxifene and aromatase inhibitors is indicated only in postmenopausal women; only tamoxifen is indicated for risk reduction of primary breast cancer in premenopausal women. | |
| Other relevant USPSTF recommendations | The USPSTF has made recommendations on screening for breast cancer and for risk assessment, genetic counseling, and genetic testing for BRCA genetic mutations. | |
The USPSTF recommends against the routine use of risk-reducing medications, such as tamoxifen, raloxifene, or aromatase inhibitors, in women who are not at increased risk for breast cancer. (D recommendation).
See the Clinical Considerations section for additional information about risk assessment.
Rationale
IMPORTANCE
Breast cancer is the most common nonskin cancer among women in the United States and the second leading cause of cancer death.1,2 The median age at diagnosis is 62 years,1 and an estimated 1 in 8 women will develop breast cancer at some point in their lifetime.2 African American women are more likely to die of breast cancer compared with women of other races.1
ASSESSMENT OF BREAST CANCER RISK STATUS
The USPSTF found convincing evidence that available risk assessment tools can predict the number of cases of breast cancer expected to develop in a population. However, these risk assessment tools perform modestly at best in discriminating between individual women who will or will not develop breast cancer over time. Overall, the USPSTF determined that the net benefit of taking medications to reduce risk of breast cancer is larger in women who have a greater risk for developing breast cancer.
POTENTIAL BENEFITS OF RISK-REDUCING MEDICATIONS
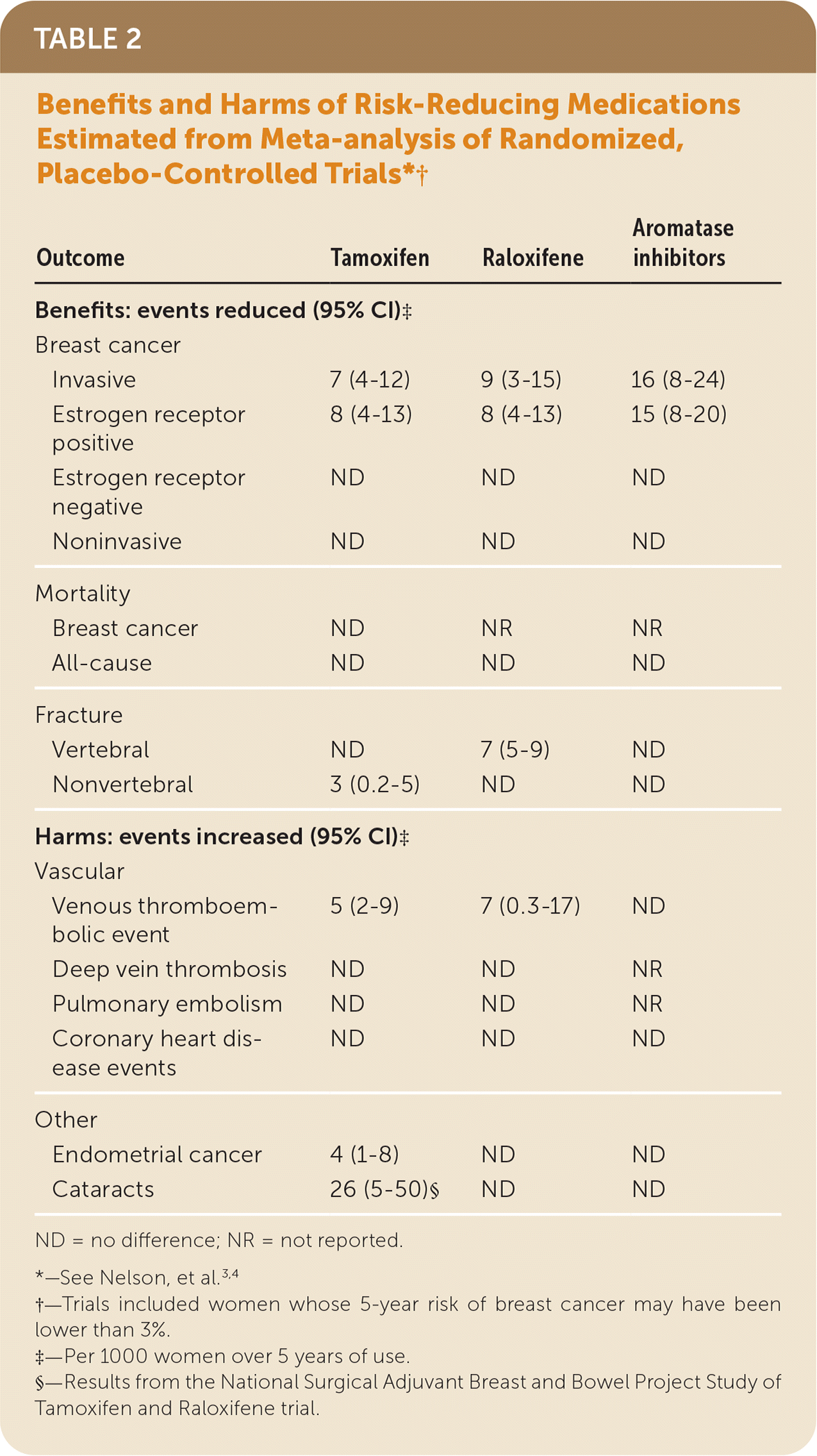
| Outcome | Tamoxifen | Raloxifene | Aromatase inhibitors |
|---|---|---|---|
| Benefits: events reduced (95% CI)‡ | |||
| Breast cancer | |||
| Invasive | 7 (4–12) | 9 (3–15) | 16 (8–24) |
| Estrogen receptor positive | 8 (4–13) | 8 (4–13) | 15 (8–20) |
| Estrogen receptor negative | ND | ND | ND |
| Noninvasive | ND | ND | ND |
| Mortality | |||
| Breast cancer | ND | NR | NR |
| All-cause | ND | ND | ND |
| Fracture | |||
| Vertebral | ND | 7 (5–9) | ND |
| Nonvertebral | 3 (0.2–5) | ND | ND |
| Harms: events increased (95% CI)‡ | |||
| Vascular | |||
| Venous thromboembolic event | 5 (2–9) | 7 (0.3–17) | ND |
| Deep vein thrombosis | ND | ND | NR |
| Pulmonary embolism | ND | ND | NR |
| Coronary heart disease events | ND | ND | ND |
| Other | |||
| Endometrial cancer | 4 (1–8) | ND | ND |
| Cataracts | 26 (5–50)§ | ND | ND |
Both tamoxifen and raloxifene can reduce risk of some types of skeletal fractures, independent from the risk of breast cancer.
The USPSTF found that the benefits of taking tamoxifen, raloxifene, and aromatase inhibitors to reduce risk for breast cancer are no greater than small in women not at increased risk for the disease.
POTENTIAL HARMS OF RISK-REDUCING MEDICATIONS
The USPSTF found convincing evidence that tamoxifen and raloxifene are associated with small to moderate harms. Tamoxifen and raloxifene increase risk for venous thromboembolic events; tamoxifen increases risk more than raloxifene (Table 23,4 ), and the potential for harms is greater in older women than in younger women. The USPSTF also found adequate evidence that tamoxifen, but not raloxifene, increases risk for endometrial cancer in women with a uterus. Tamoxifen also increases risk of cataracts. Vasomotor symptoms (hot flashes) are a common adverse effect of both medications.
The USPSTF found adequate evidence that the harms of aromatase inhibitors are also small to moderate. These harms include vasomotor symptoms, gastrointestinal symptoms, musculoskeletal pain, and possible cardiovascular events, such as stroke. Aromatase inhibitors do not reduce, and may even increase, risk of fractures.
USPSTF ASSESSMENT
The USPSTF concludes with moderate certainty that there is a moderate net benefit from taking tamoxifen, raloxifene, or aromatase inhibitors to reduce risk of invasive breast cancer in women at increased risk.
The USPSTF concludes with moderate certainty that the potential harms of taking tamoxifen, raloxifene, and aromatase inhibitors to reduce risk of breast cancer outweigh the potential benefits in women not at increased risk for the disease.
Clinicians should discuss the limitations of current clinical risk assessment tools for predicting an individual's future risk of breast cancer when discussing the benefits and harms of risk-reducing medications with women.
Clinical Considerations
PATIENT POPULATION UNDER CONSIDERATION
This recommendation applies to asymptomatic women 35 years and older, including women with previous benign breast lesions on biopsy (such as atypical ductal or lobular hyperplasia and lobular carcinoma in situ). This recommendation does not apply to women who have a current or previous diagnosis of breast cancer or ductal carcinoma in situ.
ASSESSMENT OF RISK FOR BREAST CANCER
Various methods are available to identif y women at increased risk for breast cancer, including formal clinical risk assessment tools or assessing breast cancer risk factors without using a formal tool.
Numerous risk assessment tools, such as the National Cancer Institute Breast Cancer Risk Assessment Tool,5 estimate a woman's risk of developing breast cancer over the next 5 years. There is no single cutoff for defining increased risk for all women. Women at greater risk, such as those with at least a 3% risk for breast cancer in the next 5 years, are likely to derive more benefit than harm from risk-reducing medications6 and should be offered these medications if their risk of harms is low. Some women at lower risk for breast cancer have also been included in trials documenting reduced risk for breast cancer when taking tamoxifen, raloxifene, or aromatase inhibitors.3,4 However, when balancing the harms associated with these medications, the net benefit will be lower among women at lower risk.
Alternatively, clinicians may use combinations of risk factors (including some risk factors not included in risk assessment tools but that would have permitted enrollment in some of the risk-reduction trials) to identify women at increased risk. Some examples of combinations of multiple risk factors in women at increased risk include (but are not limited to) age 65 years or older with 1 first-degree relative with breast cancer; 45 years or older with more than 1 first-degree relative with breast cancer or 1 first-degree relative who developed breast cancer before age 50 years; 40 years or older with a first-degree relative with bilateral breast cancer; presence of atypical ductal or lobular hyperplasia or lobular carcinoma in situ on a prior biopsy.
Women with documented pathogenic mutations in the breast cancer susceptibility 1 and 2 genes (BRCA1/2) and women with a history of chest radiation therapy (such as for treatment of childhood or adolescent Hodgkin or non-Hodgkin lymphoma) are at especially high risk for breast cancer. The cumulative absolute risk of developing breast cancer in a woman who received chest radiation at age 25 years increases from an estimated 1.4% at age 35 years to an estimated 29% by age 55 years,7 although this may vary by treatment regimen. Women who carry a BRCA1 mutation have a cumulative risk for breast cancer of 72% by age 80 years; women who carry a BRCA2 mutation have a 69% cumulative risk8 (compared with a 12% lifetime risk in the general population9). Women who carry the BRCA1 mutation tend to develop estrogen receptor–negative breast cancer,10 whereas women who carry the BRCA2 mutation tend to develop estrogen receptor–positive breast cancer. However, the USPSTF was not able to find sufficient evidence on the benefits and harms of risk-reducing medications in women with BRCA1/2 gene mutations or women with a history of chest radiation, and the comprehensive management of these risk factors is beyond the scope of this Recommendation Statement. Further information on comprehensive management strategies, including risk-reducing medications, for women with these conditions is available from other organizations.
Women not at increased risk for breast cancer, such as women younger than 60 years with no additional risk factors for breast cancer, or women with a low 5-year risk of breast cancer should not be routinely offered medications to reduce risk of breast cancer because the risk of harms from these medications likely outweighs their potential benefit.
Although evidence on the best interval at which to reassess risk and indications for risk-reducing medications is not available, a pragmatic approach would be to repeat risk assessment when there is a significant change in breast cancer risk factors, for instance when a family member is diagnosed with breast cancer or when there is a new diagnosis of atypical hyperplasia or lobular carcinoma in situ on breast biopsy.
When considering prescribing breast cancer risk-reducing medications, potential benefit of risk reduction of breast cancer must be balanced against the potential harms of adverse medication effects. See below for more information on potential harms from risk-reducing medications.
RISK-REDUCING MEDICATIONS
A systematic review of trials conducted for the USPSTF found that compared with placebo, tamoxifen reduced the incidence of invasive breast cancer by 7 events per 1000 women over 5 years (95% CI, 4–12), and raloxifene reduced the incidence by 9 events (95% CI, 3–15) per 1000 women over 5 years.3,4 Given that the study participants in tamoxifen vs placebo and raloxifene vs placebo trials differed with respect to breast cancer risk and age, direct comparisons of effectiveness between tamoxifen and raloxifene cannot be made based on these placebo-controlled trials. However, the large Study of Tamoxifen and Raloxifene trial, which directly compared tamoxifen with raloxifene, found that tamoxifen reduced breast cancer risk more than raloxifene after long-term follow-up3 (Table 23,4). For women with a predicted 5-year breast cancer risk of 3% or greater, the absolute benefits are likely even higher. Tamoxifen and raloxifene have been found to reduce risk for nonvertebral and vertebral fractures, respectively.3 However, use of tamoxifen and raloxifene is also associated with increased risk for venous thromboembolic events and vasomotor symptoms. Tamoxifen also increases the risk for endometrial cancer and cataracts. These risks are increased in older women, although women who have had a hysterectomy are not at risk for endometrial cancer.
Aromatase inhibitors were found to reduce the incidence of invasive breast cancer by 16 events per 1000 women over 5 years3 (Table 23,4). As with tamoxifen and raloxifene, these absolute benefits are likely even higher for women with a predicted breast cancer risk of 3% or greater. Harms of aromatase inhibitors include vasomotor symptoms, gastrointestinal symptoms, and musculoskeletal pain. Data on harms of aromatase inhibitors for the primary risk reduction of breast cancer are limited, especially long-term harms. A trend toward increased cardiovascular events (such as transient ischemic attack and cerebrovascular accident) has been observed in some aromatase inhibitor trials for treatment of women with early-stage breast cancer (or ductal carcinoma in situ).3,11,12 Younger women with no risk factors for cardiovascular disease are less likely to have a cardiovascular event with aromatase inhibitor treatment. Aromatase inhibitors do not reduce, and may even increase, risk of fractures.
Tamoxifen, raloxifene, and aromatase inhibitors all reduce primary breast cancer risk in postmenopausal women. Use of raloxifene and aromatase inhibitors is indicated only in postmenopausal women; only tamoxifen is indicated for risk reduction of primary breast cancer in premenopausal women.
DURATION OF MEDICATION USE AND PERSISTENCE OF EFFECTS
In trials, participants typically used risk-reducing medications for 3 to 5 years.3 Benefits of tamoxifen have been found to persist up to 8 years beyond discontinuation,13,14 whereas risk for venous thromboembolic events and endometrial cancer returns to baseline after discontinuation of tamoxifen.15 Data on similarly long-term persistence of effects are not available for raloxifene or aromatase inhibitors.
ADDITIONAL APPROACHES TO PREVENTION
The USPSTF has made recommendations on screening for breast cancer16 and for risk assessment, genetic counseling, and genetic testing for BRCA genetic mutations.17 The National Cancer Institute and the Centers for Disease Control and Prevention provide information about potential ways to reduce risk of cancer, including lifestyle and diet changes.18,19
USEFUL RESOURCES
The USPSTF does not endorse any particular risk prediction tool. However, the National Cancer Institute Breast Cancer Risk Assessment Tool5 and the Breast Cancer Surveillance Consortium Risk Calculator20 are based on models tested in U.S. populations and are publicly available for clinicians and patients to use as part of the process of shared, informed decision-making about taking risk-reducing medications for breast cancer. Both tools have been calibrated in U.S. populations, but their discriminatory accuracy of predicting which women will develop breast cancer may be more limited and there is no single cutoff for defining increased risk for all women.
This recommendation statement was first published in JAMA. 2019;322(9):857–867.
The “Other Considerations,” “Discussion,” “Update of Previous USPSTF Recommendation,” and “Recommendations of Others” sections of this recommendation statement are available at https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/breast-cancer-medications-for-risk-reduction1.
The USPSTF recommendations are independent of the U.S. government. They do not represent the views of the Agency for Healthcare Research and Quality, the U.S. Department of Health and Human Services, or the U.S. Public Health Service.
