
Am Fam Physician. 2021;104(1):online
Related Practice Guideline: Treatment of Chronic Obstructive Pulmonary Disease Exacerbations: Guidelines from the American Academy of Family Physicians
Related Implementing AHRQ Effective Health Care Reviews: Therapies for COPD Exacerbations in Adults
Author disclosure: No relevant financial affiliations.
Purpose: To review the evidence and provide clinical recommendations for the management of acute exacerbations of chronic obstructive pulmonary disease (COPD).
Methods: This guideline is based on a systematic review of randomized controlled trials from database inception to January 2, 2019. The target audience for the guideline includes all primary care clinicians, and the target patient population includes adults who are experiencing acute exacerbations of COPD. This guideline was developed using a modified version of the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) system, a transparent approach to evaluating the certainty of the evidence and determining the strength of recommendations.
Recommendation 1: The American Academy of Family Physicians (AAFP) recommends that clinicians prescribe antibiotics for adults with acute exacerbations of COPD to improve clinical cure and reduce clinical failure (weak recommendation, moderate quality of evidence). Choice of antibiotic should be based on patient preferences and history because there is insufficient evidence to support a preferential recommendation.
Recommendation 2: The AAFP recommends that clinicians prescribe corticosteroids for adults with acute exacerbations of COPD to reduce clinical failure (weak recommendation, low quality of evidence). There is insufficient evidence to guide the dose, route of administration, or duration of treatment.
Good Practice Point: Short-acting bronchodilators are routinely used to improve symptoms in patients with acute exacerbations of COPD (ungraded).
Guideline Scope and Purpose
The purpose of this guideline is to provide primary care– relevant recommendations for the management of acute exacerbations of chronic obstructive pulmonary disease (COPD). The target audience includes family physicians and other primary care clinicians. The target patient population is adults with acute exacerbations of COPD.
Introduction
COPD is characterized by airflow limitation and chronic respiratory symptoms, including cough and shortness of breath. It affects around 15 million people in the United States and costs more than $32 billion annually.1,2 Chronic lower respiratory tract diseases, including COPD, are the fourth leading cause of death in the United States and the third leading cause of death worldwide.3,4 Patients with COPD are at risk of acute exacerbations of symptoms resulting in the need for additional treatment. Acute exacerbations of COPD are generally characterized by increased dyspnea, increased frequency and severity of cough, and potentially increased sputum.5 Patients experiencing acute exacerbations of COPD are at increased risk of mortality and morbidity, lower quality of life, increased hospital admissions, and a progressive decline in lung function.6–13
The goals for management of acute exacerbations of COPD include symptom resolution and recovery from the exacerbation episode via improving airflow and gas exchange in addition to reducing lung inflammation. Prevention or reduction of severity of subsequent exacerbation episodes is also a goal for many management and prevention strategies. Typical interventions used for the management of COPD exacerbations include corticosteroids, systemic antibiotics, inhaled bronchodilators, and supplemental oxygen. Surprisingly, there is limited evidence for many of these treatments in this population.14 Recently, the role of mucolytics and aminophyllines has been studied based on their mechanism of action to aid in thinning and clearing of mucus and improving dyspnea, respectively.15,16
Several nonpharmacologic treatments have also been assessed for management and/or prevention of COPD exacerbations, including pulmonary rehabilitation programs, chest physiotherapy, and nutritional supplements. Chest physiotherapy includes the use of breathing techniques, vibration/percussion of the chest, and autogenic drainage (using breathing to loosen mucus). Vitamin D and omega-3 fatty acid supplements have also been highlighted as potential therapies for the prevention and management of acute exacerbations of COPD.14
Although much of the literature centers on inpatient treatment of patients with acute exacerbations of COPD, most U.S. patients with COPD are managed by primary care physicians.17 Appropriate coordination of care between subspecialists and primary care physicians is important for prevention and management of acute exacerbations of COPD.
Methods
SYSTEMATIC REVIEW
In 2019, the Agency for Healthcare Research and Quality (AHRQ) published comparative effectiveness review no. 221, Pharmacologic and Nonpharmacologic Therapies in Adult Patients With Exacerbation of COPD: A Systematic Review.18 The systematic review was conducted by the Mayo Clinic Evidence-Based Practice Center. The report was published on the AHRQ website, as well as in two additional systematic review articles.14,19
The report included studies evaluating the effectiveness of systemic antibiotics, systemic corticosteroids, and other pharmacologic and nonpharmacologic therapies. The authors also searched for studies of the effectiveness of combinations of treatments and compared different regimens of antibiotics and corticosteroids. Effectiveness of treatments was stratified by severity of disease where appropriate. The target population was defined as adults who experienced exacerbation events related to COPD. The Mayo Clinic Evidence-Based Practice Center worked with key informants and a technical expert panel consisting of stakeholders, including physician and patient representatives. Family physicians served in both groups. The report included the following key questions represented by the analytic framework in Figure 1.18
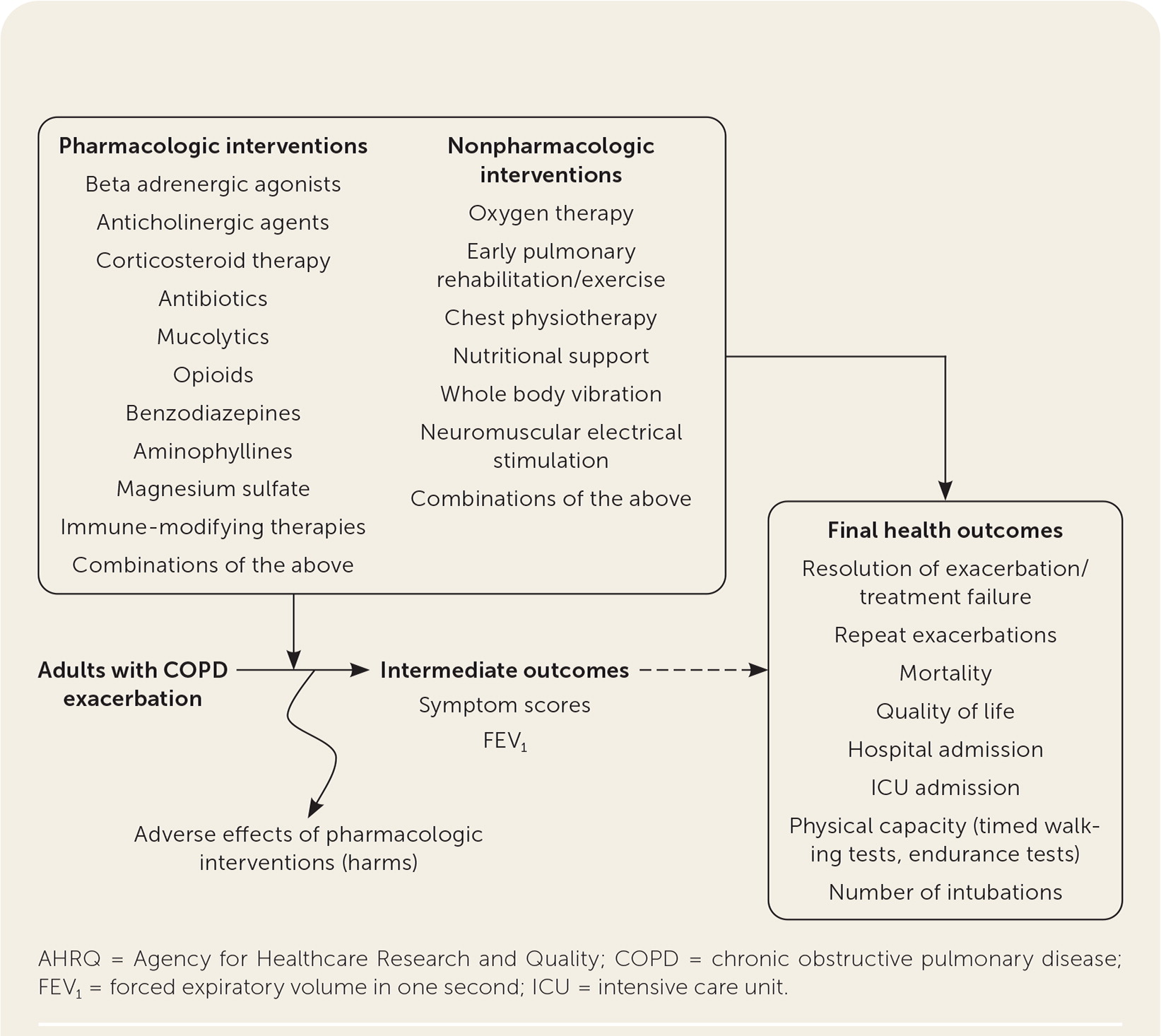
Key Question 1. In adult patients with exacerbations of COPD, what are the benefits and harms of systemic corticosteroids and antibiotics compared with placebo or standard care?
Key Question 2. In adult patients with exacerbations of COPD, what are the benefits and harms of emerging and other pharmacologic and nonpharmacologic therapies compared with placebo or standard care?
Key Question 3. In adult patients with exacerbations of COPD, what are the benefits and harms of combinations of treatments that are individually effective (based on empirical evidence in stable COPD)?
Key Question 4. In adult patients with exacerbations of COPD, what is the comparative effectiveness of different regimens of antibiotics and systemic corticosteroids based on type of agents (e.g., broad-spectrum vs. narrow-spectrum antibiotics), delivery modes (e.g., intravenous, oral), and durations of treatment?
Constructing the Guideline
The AAFP's Commission on Health of the Public and Science appointed a group to develop the guideline. Members of the guideline development group included physicians with expertise in guideline development, family medicine, internal medicine, and pulmonary medicine, in addition to a consumer representative. This group followed the guideline development process that can be found in the AAFP Clinical Practice Guideline Manual.20 The group reviewed the evidence from the AHRQ evidence report and used a modified version20 of the GRADE21 system to rate the quality of the evidence for each outcome and the overall strength of each recommendation. The strength of recommendation reflects the extent to which one can be confident that the desirable effects of an intervention outweigh the undesirable effects and reflects the degree to which there is evidence of improved patient-oriented health outcomes (Table 1).
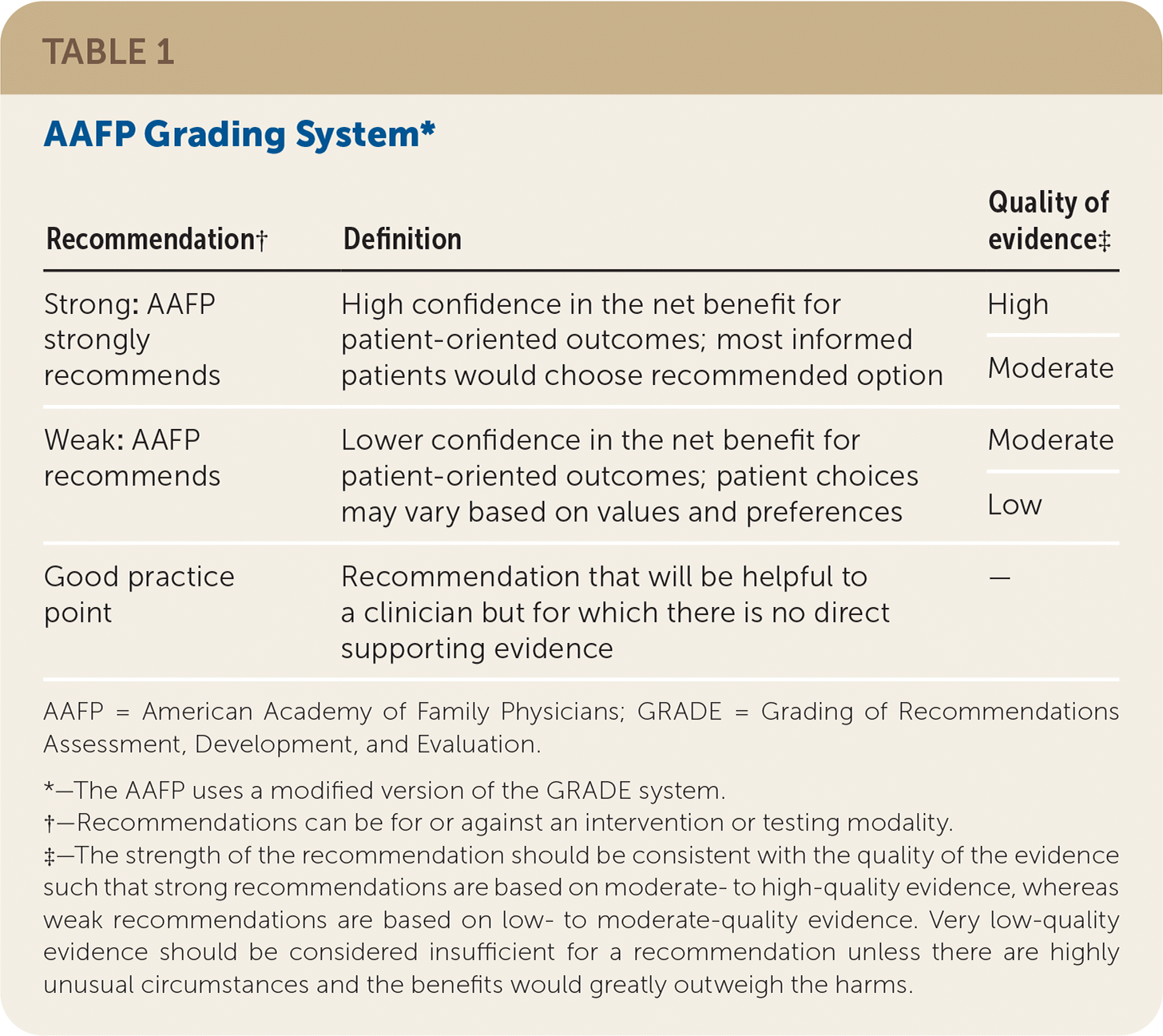
| Recommendation† | Definition | Quality of evidence‡ |
|---|---|---|
| Strong: AAFP strongly recommends | High confidence in the net benefit for patient-oriented outcomes; most informed patients would choose recommended option | High |
| Moderate | ||
| Weak: AAFP recommends | Lower confidence in the net benefit for patient-oriented outcomes; patient choices may vary based on values and preferences | Moderate |
| Low | ||
| Good practice point | Recommendation that will be helpful to a clinician but for which there is no direct supporting evidence | — |
The quality of evidence is based on the certainty of the evidence. High-quality evidence means the authors have high certainty in the estimate of effect, and additional studies most likely will not change the outcome.22,23 Low-quality evidence means that the authors have lower certainty in the estimate of effect, and additional studies will likely change the result.
The guideline development group prioritized patient-oriented clinical outcomes, which included mortality, quality of life, clinical cure, and treatment failure. Interventions, such as lung function tests, that improved intermediate outcomes but did not improve the identified patient-oriented outcomes, were not included in the recommendations. The wording of the recommendations reflects the strength and direction of the recommendation, and the quality of the evidence was listed parenthetically. Guideline recommendations were finalized based on consensus of the guideline development group after completion of GRADE evidence-to-decision frameworks, which enabled consideration of the strength of the evidence in addition to issues of feasibility, acceptability, equity, and patient preferences and values (Table 218).
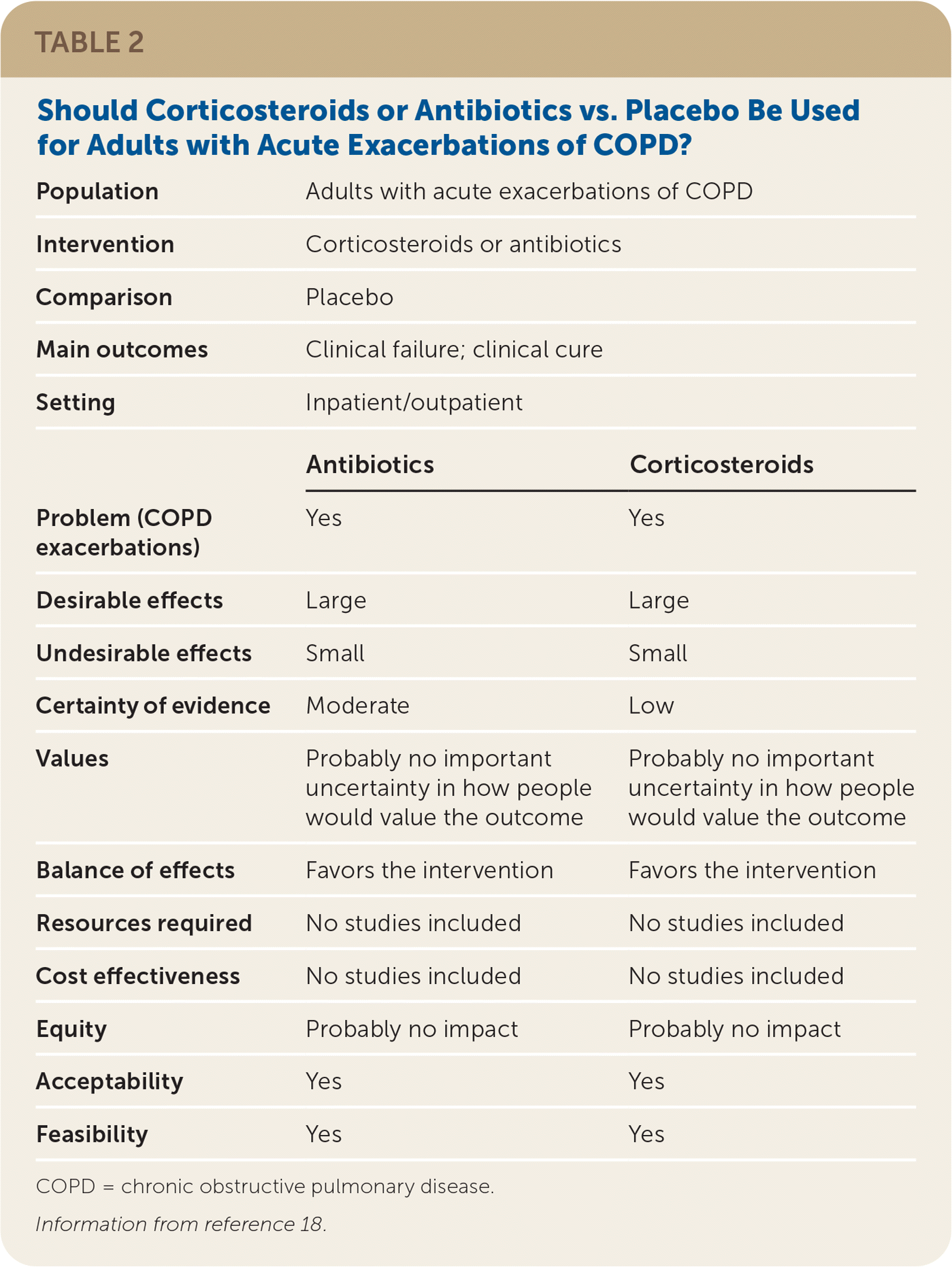
| Population | Adults with acute exacerbations of COPD | |
| Intervention | Corticosteroids or antibiotics | |
| Comparison | Placebo | |
| Main outcomes | Clinical failure; clinical cure | |
| Setting | Inpatient/outpatient | |
| Antibiotics | Corticosteroids | |
| Problem (COPD exacerbations) | Yes | Yes |
| Desirable effects | Large | Large |
| Undesirable effects | Small | Small |
| Certainty of evidence | Moderate | Low |
| Values | Probably no important uncertainty in how people would value the outcome | Probably no important uncertainty in how people would value the outcome |
| Balance of effects | Favors the intervention | Favors the intervention |
| Resources required | No studies included | No studies included |
| Cost effectiveness | No studies included | No studies included |
| Equity | Probably no impact | Probably no impact |
| Acceptability | Yes | Yes |
| Feasibility | Yes | Yes |
Patient/Consumer Perspective
Patient preferences and perspective were sought during all stages of guideline development. AHRQ routinely includes patients in the development of evidence reviews to provide input on the scope and key questions. Additionally, all AHRQ evidence reviews are available for public review and comment before being finalized. As part of the guideline development process, the AAFP included a consumer representative in the guideline development group. This member participated throughout the guideline process and was a voting member of the panel. In addition to providing insight into patient preferences during development of the recommendations, the patient representative assisted in developing the supporting text for the guideline. Patient representatives were also included in the peer-review process.
Peer Review
The guideline was peer-reviewed by relevant internal and external stakeholders, including patients and representatives from family medicine, pulmonology, and internal medicine. All comments and any modifications based on those comments were documented. The AAFP Commission on Health of the Public and Science and AAFP board of directors reviewed and approved the final version of the guideline.
Conflict of Interest
The AAFP considers both financial and intellectual conflicts of interest (COI), which were solicited in writing at the beginning of the guideline process and updated verbally at each subsequent call. No panel member disclosed any COI. Policies for disclosures and management of COI are outlined in the AAFP Clinical Practice Guideline Manual.20
Guideline Updating
All AAFP guidelines are scheduled for review five years after completion. Guidelines are reviewed at a shorter interval if new evidence becomes available. This process is managed through the Commission on Health of the Public and Science. Following review, the AAFP determines if the guideline should be reaffirmed, updated, or deleted from the website. All current guidelines developed by the AAFP are available to the public at https://www.aafp.org/patient-care/browse/type.tag-clinical-practice-guidelines.html.
Recommendations
RECOMMENDATION 1
The AAFP recommends that clinicians prescribe systemic antibiotics for adults with acute exacerbations of COPD to improve clinical cure and reduce clinical failure (weak recommendation, moderate quality of evidence). Choice of antibiotic should be based on local resistance patterns, affordability, and patient history and preferences because there is insufficient evidence to support a preferential recommendation.
Moderate quality of evidence showed that treatment of acute exacerbations with antibiotics improved the rate of clinical cure and decreased the rate of clinical failure in adult patients with COPD (Table 3).25–29 Clinical cure was defined as the complete improvement of clinical signs and symptoms following treatment. Clinical failure was defined as the lack of a significant improvement of clinical signs and symptoms and/or the requirement for additional or alternate treatment for an acute exacerbation event. Three randomized controlled trials (RCTs) with 633 patients examined the effectiveness of systemic antibiotics in the resolution (clinical cure) of an acute exacerbation of COPD compared with placebo.25–27 The pooled effect size showed increased rates of clinical cure at the end of the intervention (odds ratio [OR] = 2.03; 95% CI, 1.47 to 2.80) compared with placebo. This benefit was observed independently of the level of severity of the exacerbation episode and of treatment setting (outpatient vs. hospital).14
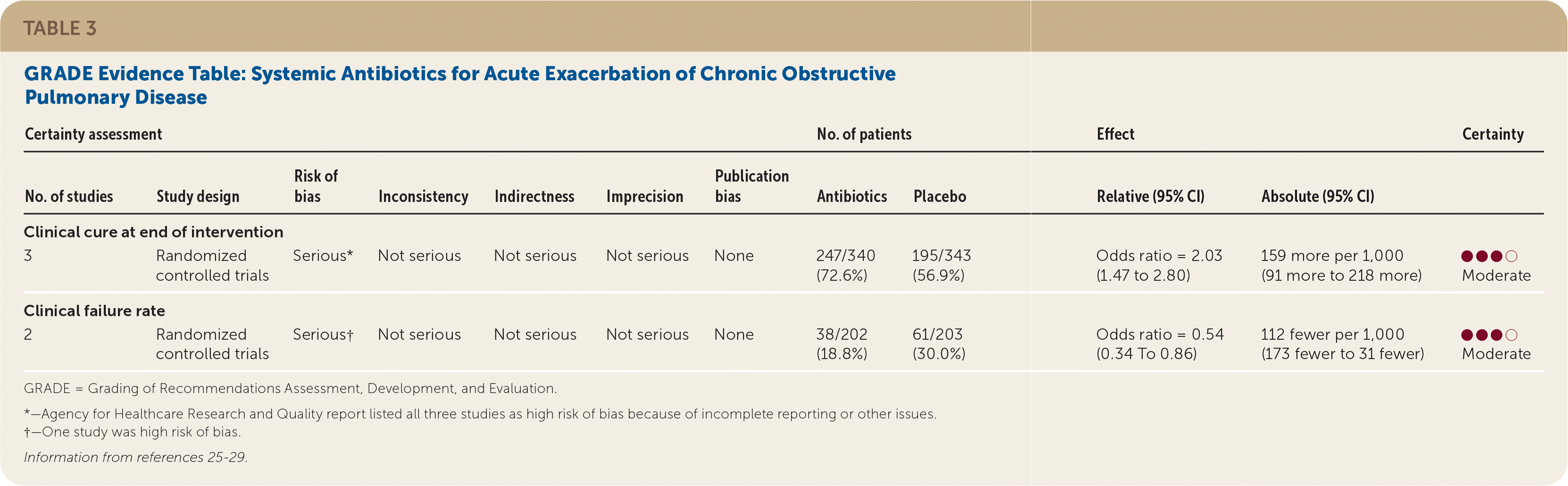
| Certainty assessment | No. of patients | Effect | Certainty | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of studies | Study design | Risk of bias | Inconsistency | Indirectness | Imprecision | Publication bias | Antibiotics | Placebo | Relative (95% CI) | Absolute (95% CI) | |
| Clinical cure at end of intervention | |||||||||||
| 3 | Randomized controlled trials | Serious* | Not serious | Not serious | Not serious | None | 247/340 (72.6%) | 195/343 (56.9%) | Odds ratio = 2.03 (1.47 to 2.80) | 159 more per 1,000 (91 more to 218 more) | ●●●○Moderate |
| Clinical failure rate | |||||||||||
| 2 | Randomized controlled trials | Serious† | Not serious | Not serious | Not serious | None | 38/202 (18.8%) | 61/203 (30.0%) | Odds ratio = 0.54 (0.34 To 0.86) | 112 fewer per 1,000 (173 fewer to 31 fewer) | ●●●○Moderate |
One study from the Netherlands randomized 223 patients to either 200 mg of doxycycline or placebo for seven days in addition to corticosteroids.25 At the end of 10 days, patients receiving doxycycline showed higher rates of clinical cure compared with those receiving placebo. A second study randomized 100 patients to receive 500 mg of a quinolone or 500 mg of amoxicillin compared with placebo for 10 days.27 No significant differences were observed between the treatment and placebo groups. The third trial was conducted in Spain and randomized 310 patients to receive amoxicillin or placebo.26 Patients treated with amoxicillin had a significantly longer time to subsequent exacerbations than those receiving placebo, which was considered a measure of clinical cure.
Two RCTs with a total of 505 patients showed less treatment failure for acute exacerbations with systemic antibiotics compared with placebo (OR = 0.54; 95% CI, 0.34 to 0.86).28,29 The evidence for these outcomes was rated as moderate quality because of the risk of bias for four out of the five included studies.
There were no statistically significant differences in other outcomes of interest, including mortality, quality of life, and adverse events. Although studies were found that examined different types and dosages of antibiotics, there was insufficient evidence to estimate an effect on most of the outcomes of interest.18 Similarly, no studies were found that examined routes of administration of antibiotics for this population.18 Clinicians should base decisions on type, duration, route, and cost of antibiotics for resolution of acute exacerbations of COPD based on local resistance patterns, affordability, and patient history and preferences.
RECOMMENDATION 2
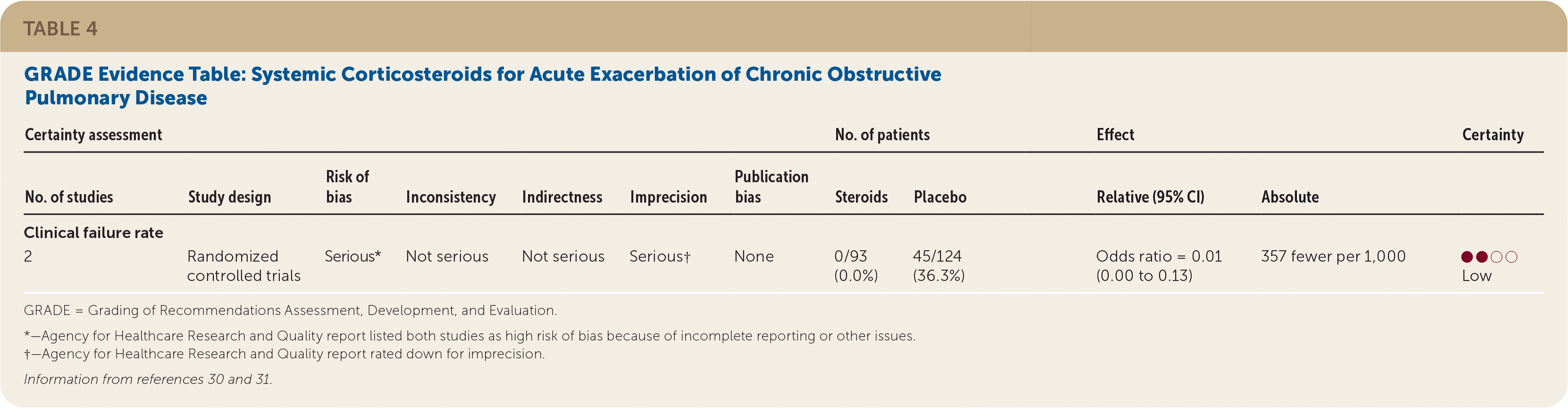
The AAFP recommends that clinicians prescribe corticosteroids for adults with acute exacerbations of COPD to reduce clinical failure (weak recommendation, low quality of evidence). There is insufficient evidence to guide the dose, route of administration, or duration of treatment.
Low quality of evidence demonstrated that systemic corticosteroids decreased the clinical failure rate in adults with acute exacerbations of COPD (Table 4).30,31 Two RCTs with a total of 217 patients showed less treatment failure for acute exacerbations with systemic corticosteroids compared with placebo (OR = 0.01; 95% CI, 0.00 to 0.13).30,31
| Certainty assessment | No. of patients | Effect | Certainty | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of studies | Study design | Risk of bias | Inconsistency | Indirectness | Imprecision | Publication bias | Steroids | Placebo | Relative (95% CI) | Absolute | |
| Clinical failure rate | |||||||||||
| 2 | Randomized controlled trials | Serious* | Not serious | Not serious | Serious† | None | 0/93 (0.0%) | 45/124 (36.3%) | Odds ratio = 0.01 (0.00 to 0.13) | 357 fewer per 1,000 | ●●○○Low |
One RCT conducted in the Veterans Affairs health system randomized 190 patients to receive a course of intravenous methylprednisolone and oral prednisone or to receive placebo for eight weeks.30 A third group, which was not included in the analysis, received corticosteroids for two weeks. Patients receiving placebo had significantly higher rates of clinical failure than those receiving corticosteroids (33% vs. 0%). The second study looked at 26 patients who received oral prednisone or placebo.31 No treatment failures were observed in the treatment group (n = 13) compared with 61% of those in the placebo group (n = 14). There was no significant difference at longest follow-up (one month) for clinical failure as reported in the AHRQ evidence review.14 This evidence was rated as low quality because of the risk of bias and imprecision in both studies.18 The improvement was observed independent of severity of exacerbation episode and treatment setting.
There were no differences observed for other outcomes of interest, including mortality and rehospitalization. An increase in the number of adverse events was observed with systemic corticosteroid treatment, but it was not statistically significant.18
Three studies compared the effectiveness of different durations of systemic corticosteroids (three vs. 10 days, five vs. 14 days, and two vs. eight weeks) in the inpatient setting. No statistically significant differences in outcomes of interest were observed. Interestingly, these data suggest that five days of treatment is not inferior to 14 days of treatment.14 This is consistent with a large observational study that was published after the search dates for the evidence review.32 This study used administrative data and included 10,152 patient records. These data showed small increases in rates of pneumonia-associated hospitalization and mortality associated with longer courses of corticosteroids.32
There was insufficient evidence to guide decisions about routes of administration (oral, inhaled, or intravenous) of corticosteroids in this population.18 One study examining oral with intravenous administration showed no differences in outcomes; however, this study was found to have a high risk of bias and severe imprecision and was deemed to provide insufficient evidence.33
GOOD PRACTICE POINT
Short-acting bronchodilators are routinely used to improve symptoms in patients with acute exacerbations of COPD (ungraded).
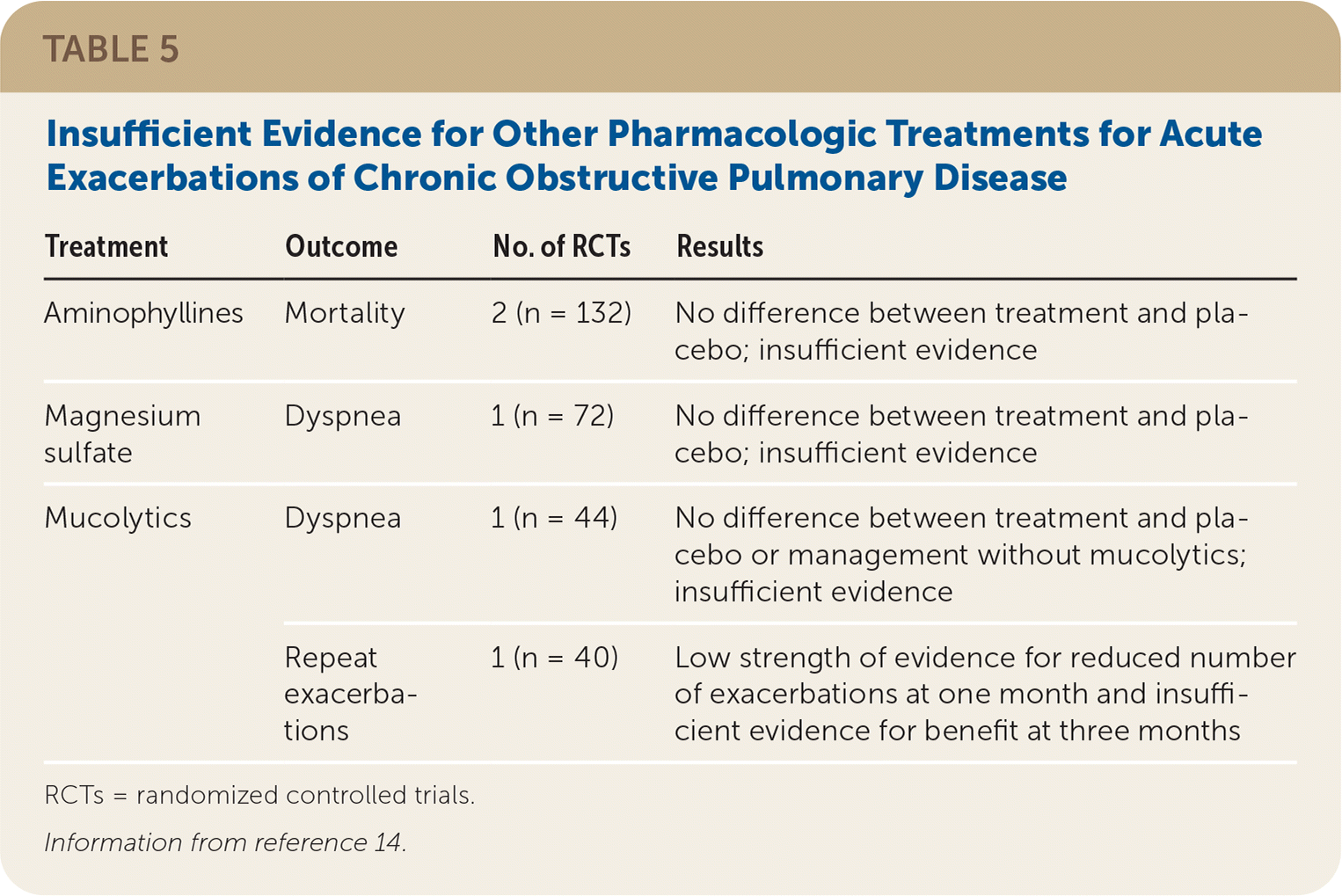
The evidence report evaluated other pharmacologic treatments and dietary supplements for acute exacerbations of COPD. However, there was insufficient evidence for all therapies other than antibiotics and corticosteroids for the outcomes of interest. In particular, there were no high-quality RCTs demonstrating the benefit of short-acting bronchodilators for the treatment of acute exacerbations of COPD. Three studies were included in the review that looked at the comparative effectiveness of bronchodilators. One study compared ipratropium (Atrovent) with salbutamol (albuterol),34 two studies compared ipratropium/salbutamol (Combivent) with salbutamol alone,34,35 and a third study compared salbutamol plus inhaled corticosteroids with fenoterol (not available in the United States).36 None of the studies looked at patient-oriented outcomes, and no significant differences were observed in forced expiratory volume in one second or adverse events.14
There are limited data, specifically placebo-controlled trials, supporting the role of short-acting bronchodilators for acute exacerbations of COPD. However, these agents, specifically beta-2 agonists, anticholinergic agents, or combinations, are routinely recommended, primarily for symptom control.37–40 Because of the widespread recommendation for and use of these medications, the AAFP identifies the use of short-acting bronchodilators for acute exacerbations of COPD as a good practice point. As discussed later, this area has been identified as a research gap, and the AAFP calls for more research to address the role of these medications.
| Treatment | Outcome | No. of RCTs | Results |
|---|---|---|---|
| Aminophyllines | Mortality | 2 (n = 132) | No difference between treatment and placebo; insufficient evidence |
| Magnesium sulfate | Dyspnea | 1 (n = 72) | No difference between treatment and placebo; insufficient evidence |
| Mucolytics | Dyspnea | 1 (n = 44) | No difference between treatment and placebo or management without mucolytics; insufficient evidence |
| Repeat exacerbations | 1 (n = 40) | Low strength of evidence for reduced number of exacerbations at one month and insufficient evidence for benefit at three months |
Nonpharmacologic Treatments
The evidence review found only a few studies evaluating nonpharmacologic treatments for acute exacerbations of COPD, resulting in insufficient evidence for patient-oriented outcomes prioritized by the guideline development group.18 Three studies on chest physiotherapy were included, showing no significant statistical differences between therapy and no therapy for mortality, dyspnea, or quality of life.41–43 Resistance training may improve dyspnea and quality of life; however, there was only a single study of 60 patients included in the review.44 Aerobic training was associated with a small improvement in dyspnea at the end of the intervention for patients with acute exacerbations of COPD, but this was a single study with 46 patients and was rated as high risk of bias, so additional research is needed.45 A single study on whole body vibration showed that it may improve quality of life, but this was considered low-quality evidence with a single RCT of 49 patients.46
The review included a fairly large trial with 214 patients comparing titrated oxygen with high-flow oxygen, which found a reduction in mortality in patients with acute exacerbations of COPD who were given titrated oxygen.47 This study was rated as high risk of bias resulting in a low strength of evidence for this outcome. However, this result is similar to other observations associating increased risk of harms with high-flow oxygen when used to maintain higher saturation levels.47–49
Various dietary modifications and supplements were also included in the evidence review, but there was limited evidence to recommend for or against these treatments.
Implementations of Recommendations
BARRIERS TO IMPLEMENTATION OF THE GUIDELINE INTO CLINICAL PRACTICE
Most studies included in the evidence report evaluated patients who were hospitalized and experiencing severe acute exacerbations of COPD, limiting the applicability to outpatient settings and patients with less severe disease. Additionally, although there was insufficient evidence to draw conclusions among different antibiotics, the choice of treatment will depend on local antibiotic resistance patterns, which vary among patients, locations, and points in time.18 Shared decision-making is important in determining treatment plans. Any patient education materials should be written at an appropriate literacy level with risks and benefits of treatment conveyed adequately.
Addressing potential disparities that exist across racial, socioeconomic, and geographic demographics should be considered when implementing any guideline recommendations. Racial and gender disparities have been observed in frequency of exacerbations, health outcomes, and use of health care services.17 Lack of access to health care services, in addition to other social determinants of health that affect patients' ability to obtain services, could be a significant impediment to implementation of these recommendations. Practices should consider screening for social determinants of health and help address those issues by developing and maintaining a directory of resources for patients.50 Increasingly, private and governmental insurers are employing case/care managers to assist patients and clinicians in obtaining the additional resources needed for supporting patients who face challenging situations. Clinicians may also consider the availability of telemedicine resources for counseling and follow-up for patients with acute exacerbations of COPD.
TECHNIQUES FOR IMPLEMENTATION
Chronic care management can be effectively implemented by primary care practices to manage patients with chronic conditions, such as diabetes mellitus, cardiovascular disease, and COPD. It is a model that has been used to educate and empower patients to track, prevent, and self-manage symptoms, leading to earlier reporting of COPD exacerbations to primary care physicians and reducing use of the emergency department.51
Telemedicine may provide additional assistance in management of patients with COPD exacerbations. More studies are needed to determine the full impact on outcomes for patients with COPD; to date, only one study has been found that included patients with acute exacerbations who were managed using telemedicine. However, given the onset of widespread use of telemedicine in 2020, there may be additional support for its use in this patient population.52 Another study assessed the effectiveness of an integrative approach with both telemedicine and discharge bundles. Patients in the integrative approach group had lower rates of hospital admissions and lower rates of readmission to the hospital for exacerbation episodes.53
For acute treatment, integration of guideline recommendations for order sets have been used to standardize treatment of patients who present with acute exacerbations of COPD.54 Order sets were shown to reduce length of hospital stay and readmissions.55,56 Additionally, standard order sets resulted in reduced errors in prescribing corticosteroids and antibiotics.54,55
Discharge bundles have also been used to provide additional patient education and other interventions to reduce hospital readmissions in patients with COPD. A 2017 systematic review assessed the effectiveness of different discharge bundles.57 A total of 14 studies were included covering a range of interventions such as self-management education, assessment/referral for pulmonary rehabilitation, arrangement of outpatient follow-up, and referral to smoking cessation programs. The bundles were associated with a decrease in hospital readmissions.57 Coordination between sub-specialists or other clinicians providing in-patient care (hospitalists, emergency physicians) and the primary care physician is crucial in management of patients with chronic conditions.
Several services offered routinely in primary care are effective in preventing exacerbations of COPD, including smoking cessation counseling and immunizations. Hospitalization for acute exacerbations may serve as an opportunity to provide interventions for behavior change, such as smoking cessation counseling.58 Screening, brief interventions, and referral to treatment have been used successfully to address risky alcohol use and are promoted as part of the recommendation from the U.S. Preventive Services Task Force to aid in smoking cessation.59 For patients with COPD, interventions aimed at smoking cessation were shown to be effective in increasing quit rates and sustaining abstinence from tobacco use.60 Similarly, providing immunizations against pneumococcal infections and influenza was shown to reduce the number of exacerbations in patients with COPD.61,62 Additionally, potential comorbidities can be effectively addressed in the primary care setting. If a patient has not had a spirometry test previously, this can be administered by the primary care physician to confirm that other conditions, such as asthma or restrictive lung disease, are not contributing to the exacerbation episodes.
Limitations of the Guideline
The guideline development group acknowledges that there were several limitations in evidence used to inform the guideline recommendations, including:
Small number of studies and small number of patients in many studies
Variability in the reporting of outcomes with a limited number that were considered patient-oriented
Most studies were in hospital settings and in patients with severe acute exacerbations of COPD, limiting applicability to outpatient settings and patients with mild or moderate events
Conclusions and Future Research
The purpose of this updated guideline is to provide clinical recommendations for primary care physicians to treat patients who are experiencing acute exacerbations of COPD. The management of stable COPD was considered outside the scope of this guideline but is addressed elsewhere.37,38,40 The AAFP recommendations for managing acute exacerbations of COPD are consistent with guidance from others.37,38 Both systemic antibiotics and corticosteroids were observed to significantly improve symptom resolution in this population as measured by increased clinical cure rates. Treatment with antibiotics also decreased clinical failure rate. Treatment decisions should be based on clinical judgment and patient preferences and values, and involve shared decision-making by the patient and clinician.
This guideline was developed using available evidence; however, significant gaps were identified in the AHRQ systematic review and by members of the guideline development group. New research into these areas may affect the recommendations, at which time the guideline will be updated accordingly. Research that would provide important information for the clinical questions discussed in this guideline includes the following:
Studies including patient-oriented outcomes, such as clinical cure/clinical failure, quality of life, and repeat exacerbations
Most of the studies included in the evidence report relied on the intermediate outcomes of lung function to assess effectiveness of the different treatments
Studies examining the effectiveness of treatments in subpopulations that may receive disparate treatment (e.g., sex, age, ethnicity) and in different settings (e.g., outpatient) and with different severities of exacerbation
Studies for the efficacy and comparative effectiveness of bronchodilators to determine the role of different medications and combinations of medications for patients experiencing acute exacerbations of COPD
There are limited data, specifically placebo-controlled trials, supporting the role of short-acting bronchodilators for acute exacerbations of COPD. Most trials have focused on delivery methods for these medications, but there is insufficient evidence to determine the relative benefits and harms of single or combination short-acting bronchodilator therapy in the various care settings and presentations of patients with acute exacerbations of COPD. Additionally, studies are needed to determine the role of rapid-onset, long-acting bronchodilators in acute exacerbations of COPD.
Additional studies to determine the optimal type of antibiotic and route and duration of corticosteroid treatment
Further research is required to determine if specific classes of antibiotics are more effective at resolving acute exacerbations of COPD. Additionally, studies are needed to determine the optimal route of administration for systemic corticosteroids, particularly around the use of oral or inhaled corticosteroids compared with intravenous corticosteroids.
Studies on the role of rapid testing of inflammatory and other markers (such as C-reactive protein [CRP]) in treatment decisions for COPD
Two trials examining the role of CRP on antibiotic prescriptions for patients who have acute exacerbations of COPD were published after the dates of the literature search for the AHRQ report. Both trials suggest that using CRP point-of-care testing to guide antibiotic treatment could significantly reduce antibiotic use for COPD exacerbations without worsening outcomes.63,64
Another trial examining the role of eosinophil counts to guide corticosteroid therapy in patients who have acute exacerbations of COPD was identified. The trial included 318 patients and showed slight decreases in days of corticosteroid use without worsening outcomes such as hospitalizations or treatment failure.65
Studies examining the effectiveness of nonpharmacologic and dietary interventions on patient-oriented outcomes in those with acute exacerbations of COPD
There were no studies identified that looked at the impact of nonpharmacologic treatments such as chest physiotherapy, transcutaneous electrical nerve stimulation, or dietary interventions on the outcomes of clinical resolution or repeat exacerbations. Chest physiotherapy is commonly prescribed in patients who are hospitalized for COPD exacerbations, but the evidence report found insufficient evidence of benefit.
Studies to determine which patients with acute exacerbations of COPD will benefit from oxygen therapy, as well as the best delivery mechanism(s) and optimal titration for oxygen therapy
There are limited data showing that the use of lower oxygen saturation targets for oxygen therapy (88% to 92%) improved mortality in patients with acute exacerbations of COPD.47,66 However, further research could identify the best delivery mechanism for oxygen, as well as methods for appropriate titration and monitoring of therapy.
Data Sources: Databases were searched for English-language studies published from database inception to January 2, 2019, and included Embase; epub ahead of print, in-process, and other non-indexed citations; MEDLINE Daily; MEDLINE; Cochrane Central Register of Controlled Trials; Ovid Cochrane Database of Systematic Reviews; and Scopus. Additional databases searched included the U.S. Food and Drug Administration, ClinicalTrials.gov, Health Canada, Medicines and Healthcare Products Regulatory Agency, AHRQ's Horizon Scanning System, conference proceedings, patient advocate group websites, and medical society websites. Titles and abstracts were reviewed using prespecified inclusion and exclusion criteria.18 The Mayo Clinic Evidence-Based Practice Center then assessed all included studies for quality using established methodology.67 The report included 98 RCTs (13,401 patients, mean treatment duration = 9.9 days, mean follow-up = 3.7 months).18 A targeted, updated literature search to identify new RCTs was completed. The updated search resulted in 47 articles spanning the time of the completion of the AHRQ report in 2019 through March 12, 2021. Only three reports met inclusion criteria and were considered by the panel; however, they did not impact the recommendations and were identified as areas for future research. Additionally, one updated clinical practice guideline with recommendations for acute exacerbations was identified and acknowledged in the supporting text.
Dr. Stevermer is a member of the U.S. Preventive Services Task Force (USPSTF). This article does not necessarily represent the views and policies of the USPSTF. Dr. Lin is deputy editor for AFP.
All costs associated with the development of this guideline came exclusively from the operating budget of the AAFP.
APPROVED BY THE AAFP BOARD OF DIRECTORS APRIL 2021
Disclaimer: These recommendations are provided only as assistance for clinicians making clinical decisions regarding the care of their patients. As such, they cannot substitute for the individual judgment brought to each clinical situation. As with all clinical reference resources, they reflect the best understanding of the science of medicine at the time of publication, but they should be used with the clear understanding that continued research may result in new knowledge and recommendations. All AAFP guidelines are scheduled for review five years after completion or earlier if new evidence becomes available.
