
Am Fam Physician. 2021;104(6):online
Author disclosure: No relevant financial affiliations.

Details for This Review
Study Population: 11,305 adults from 42 randomized controlled trials comparing a probiotic with placebo, alternative probiotic dose, alternative probiotic strain, or no treatment in patients receiving antibiotics
Efficacy End Points: Reduction in antibiotic-associated diarrhea (AAD)
Harm End Points: Adverse events
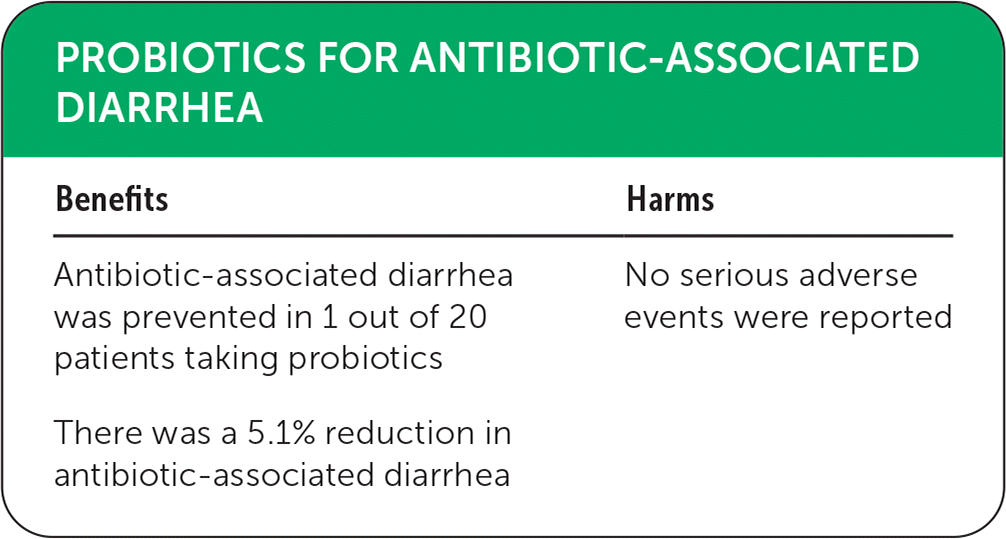
| Benefits | Harms |
|---|---|
| Antibiotic-associated diarrhea was prevented in 1 out of 20 patients taking probiotics | No serious adverse events were reported |
| There was a 5.1% reduction in antibiotic-associated diarrhea |
Narrative: AAD can occur in up to 35% of patients who receive antibiotics and is associated with higher health care costs and increased morbidity and mortality.1–6 Probiotics consist of live microbes and can improve host-microbial balance and reduce pathogenic bacteria colonization.7 A Cochrane review found that probiotics are protective against Clostridioides difficile–associated diarrhea; however, C. difficile represents only a small proportion of AAD cases.8,9 The meta-analysis summarized in this Medicine by the Numbers evaluated whether probiotics reduce the risk of AAD among adults receiving antibiotics.10
The meta-analysis included 42 randomized controlled trials with 11,305 adults receiving antibiotics of any duration and for any indication. Authors included any strain, dose, or formulation (tablets, powder, yogurt, or fermented milk drink) of probiotic. The included trials evaluated probiotic use compared with placebo, an alternative dose of probiotic (high vs. low dose), an alternative probiotic strain, or no treatment for the prevention of AAD.10 The authors excluded studies that evaluated children and used probiotics for treatment, rather than prevention, of AAD. The probiotic duration was reported in 40 out of 42 studies and ranged from five to 56 days, most commonly for the duration of antibiotic therapy plus seven days.
The primary outcome was the incidence of AAD, as defined by individual trials, during antibiotic treatment or follow-up phases. Secondary objectives were dose-specific and species-specific responses in reducing AAD.
Probiotics reduced the risk of AAD overall (13.7% vs. 18.8%; absolute risk difference = 5.1%; number needed to treat = 20; risk ratio [RR] = 0.63; 95% CI, 0.54 to 0.73; moderate-quality evidence). Subgroup analysis found that higher doses of the same probiotic had a positive protective effect compared with lower doses (RR = 0.54; 95% CI, 0.38 to 0.76). Certain species were found to be effective overall: Lactobacillus (RR = 0.63; 95% CI, 0.52 to 0.76), Saccharomyces boulardii (RR = 0.63; 95% CI, 0.46 to 0.86), Bifidobacterium animalis subsp lactis (RR = 0.70; 95% CI, 0.54 to 0.91), Bacillus licheniformis (RR = 0.39; 95% CI, 0.19 to 0.79), Bifidobacterium longum (RR = 0.46; 95% CI, 0.28 to 0.73), Bacillus subtilis (RR = 0.35; 95% CI, 0.19 to 0.62), and Bacillus clausii (RR = 0.61; 95% CI, 0.41 to 0.89). Probiotic use in patients at moderate (11% to 30%) and high (31% or greater) baseline risk of AAD demonstrated a significant overall reduction (RR = 0.61; 95% CI, 0.48 to 0.78, and RR = 0.55; 95% CI, 0.46 to 0.66, respectively). No serious adverse events were reported in the included studies.
Caveats: The meta-analysis had several limitations. There was significant heterogeneity in individual study designs, populations, illness severity, infection type, and antibiotic and probiotic administration. Although higher doses of probiotics showed a greater benefit in preventing AAD in four studies, it is difficult to interpret this effect or make comparisons because the studies used different probiotic doses. Many studies incorporated blends of species and strains for probiotic therapy. B. licheniformis, B. longum, and B. subtilis demonstrated the greatest effect sizes compared with other probiotics. Lactobacillus acidophilus, Lactobacillus casei, and S. boulardii demonstrated a moderate effect and were used in many of the included studies. Several of the studies did not provide adequate details on random sequence generation and allocation concealment. There were insufficient reporting data to perform subgroup analyses based on antibiotic class, name, administration route, dose, or duration of therapy.
Despite significant limitations due to heterogeneity, this meta-analysis found that probiotics reduced the incidence of AAD with no observed serious adverse effects. We have assigned a color recommendation of green (benefits greater than harms) for this treatment; however, the benefits of probiotics could be affected by several factors, such as the type of probiotic used or the baseline risk of AAD. Further studies are needed to compare individual probiotic types, durations, and doses and the impact of probiotics on different antibiotics.
