
Am Fam Physician. 2022;105(1):24-32
Patient information: See related handout on cluster headaches, written by the authors of this article.
Author disclosure: No relevant financial relationships.
Cluster headache, the most common form of trigeminal autonomic cephalgia, is a rare primary headache disorder that affects less than 1% of the population. The mean age of onset is 30 years, and it is two to three times more common in males. Cluster headache consists of attacks of severe unilateral pain located in the orbital, supraorbital, and/or temporal region that occur from every other day up to eight times per day and last from 15 to 180 minutes. The pain is associated with ipsilateral autonomic symptoms (most commonly lacrimation, conjunctival injection, nasal congestion or rhinorrhea, ptosis, edema of the eyelid, sweating of the forehead or face, and miosis) and a sense of agitation or restlessness. Attacks occur in clusters, called bouts, and are episodic or chronic. Common triggers include alcohol, nitroglycerin, food containing nitrates, and strong odors. Abortive treatments include triptans and oxygen; transitional treatments include steroids and suboccipital steroid injections; and prophylactic treatments include verapamil, lithium, melatonin, and topiramate. Newer treatments for cluster headache include galcanezumab, neurostimulation, and somatostatin receptor agonists.
Cluster headache is the most common form of trigeminal autonomic cephalgia, and it affects less than 1% of the population. It is characterized by attacks of severe unilateral pain located in the orbital, supraorbital, and/or temporal region. This article provides a review of patient-oriented evidence to guide the diagnosis and management of cluster headache.
| Clinical recommendation | Evidence rating | Comments |
|---|---|---|
| A sense of restlessness is one key feature of cluster headache and should be elicited in the history.1,3 | C | Consensus statement |
| First-line abortive therapies for cluster headache include 100% supplemental oxygen, subcutaneous sumatriptan (Imitrex), sumatriptan nasal spray, and intranasal zolmitriptan (Zomig).16,19–22,24–26 | A | Good quality meta-analyses and randomized controlled trials |
| Suboccipital steroid injection may be considered for transitional therapy to provide time for prophylactic therapy to take full effect.14,32,33 | A | Good quality randomized controlled trials |
| First-line prophylactic therapy for cluster headache is verapamil.12,34,37 | A | Good quality randomized controlled trials |
Epidemiology and Risk Factors
Typical age of onset is 20 to 40 years1,2; it is two to three times more common in men than women.3
Lifetime prevalence is 124 per 100,000.4
Having a first-degree relative with cluster headache increases the patient's risk five to 18 times, whereas having a second-degree relative with cluster headache increases the risk one to three times.5
Diagnosis
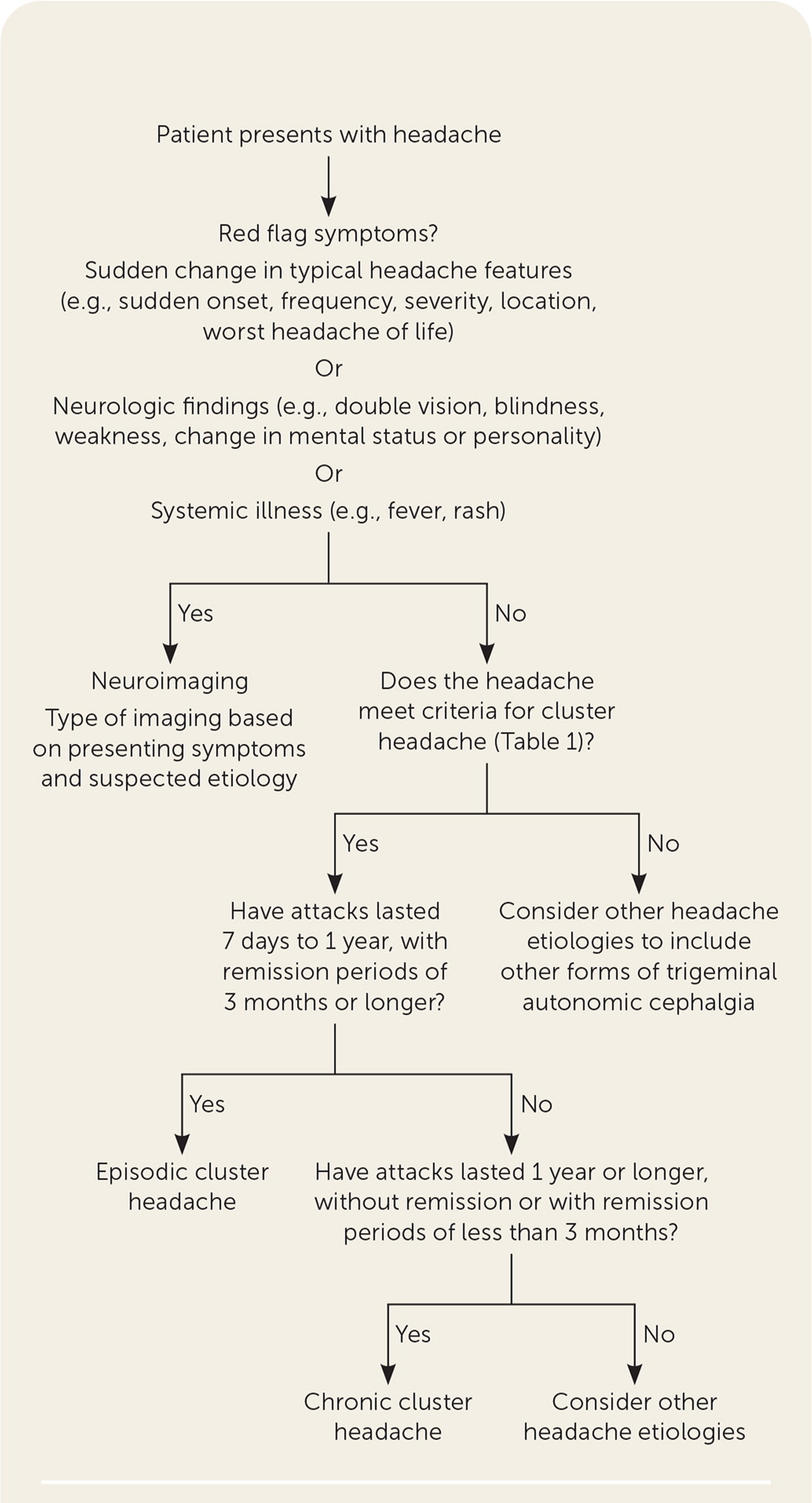
Cluster headache is a clinical diagnosis based on the criteria described in Table 1.2
Cluster headache patterns are episodic or chronic. Headache episodes that last seven days to one year with remission periods lasting three months or longer are episodic. When episodes last for one year or longer with remission periods of less than three months, they are categorized as chronic.1
A suggested approach to the evaluation of acute headache is summarized in Figure 1.1,2,6
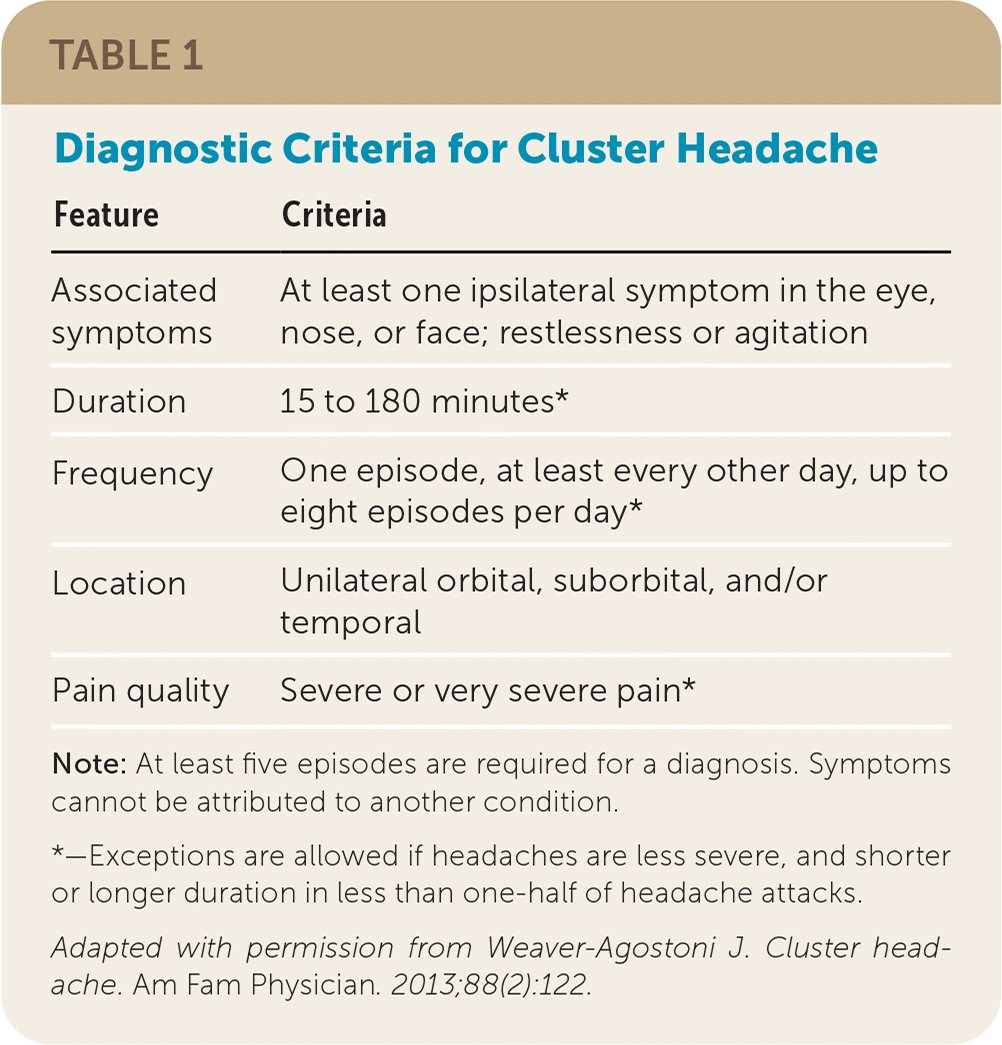
| Feature | Criteria |
|---|---|
| Associated symptoms | At least one ipsilateral symptom in the eye, nose, or face; restlessness or agitation |
| Duration | 15 to 180 minutes* |
| Frequency | One episode, at least every other day, up to eight episodes per day* |
| Location | Unilateral orbital, suborbital, and/or temporal |
| Pain quality | Severe or very severe pain* |
DIFFERENTIAL DIAGNOSIS
The differential diagnosis of cluster headache includes other trigeminal autonomic cephalgias such as paroxysmal hemicrania, short-lasting unilateral neuralgiform headache attacks with conjunctival injection and tearing, short-lasting unilateral neuralgiform headache attacks with cranial autonomic symptoms, hemicrania continua, or secondary cluster headache caused by intracranial structural lesions (e.g., pituitary adenoma).1,7
Trigeminal neuralgia typically affects the second and third branches of the trigeminal nerve, whereas the first branch is affected in cluster headache.1,7
Migraine headaches are often accompanied by other symptoms that are not typical of cluster headache, including photophobia, phonophobia, and nausea or vomiting. Most patients with migraine find movement worsens their headache, whereas patients with cluster headache can feel agitated and restless.1,7
HISTORY AND PHYSICAL EXAMINATION
The typical presentation of cluster headache is unilateral severe pain in the orbital, supraorbital, and/or temporal region and is associated with one or more ipsilateral autonomic symptoms, including conjunctival injection, lacrimation, rhinorrhea, nasal congestion, miosis and/or ptosis, eyelid edema, and facial sweating.1,7 Another key symptom is a sense of restlessness.1,3,7
A cluster headache can last from 15 to 180 minutes when untreated and occurs up to eight times per day in a long-term pattern.1
Triggers include medications (vasodilators) such as nitroglycerin, histamine release, tobacco exposure, alcohol, foods that contain nitrates, nail varnish, and petroleum.1,2
DIAGNOSTIC TESTING
Neuroimaging with computed tomography or magnetic resonance imaging is not routinely recommended.
Imaging should be performed in patients with sudden changes in headache features (e.g., sudden onset, frequency, severity, location, worst headache of the patient's life), presence of neurologic findings (e.g., double vision, blindness, weakness, change in mental status or personality), or systemic illness (e.g., fever, rash).1,2,6
Treatment
ABORTIVE THERAPY
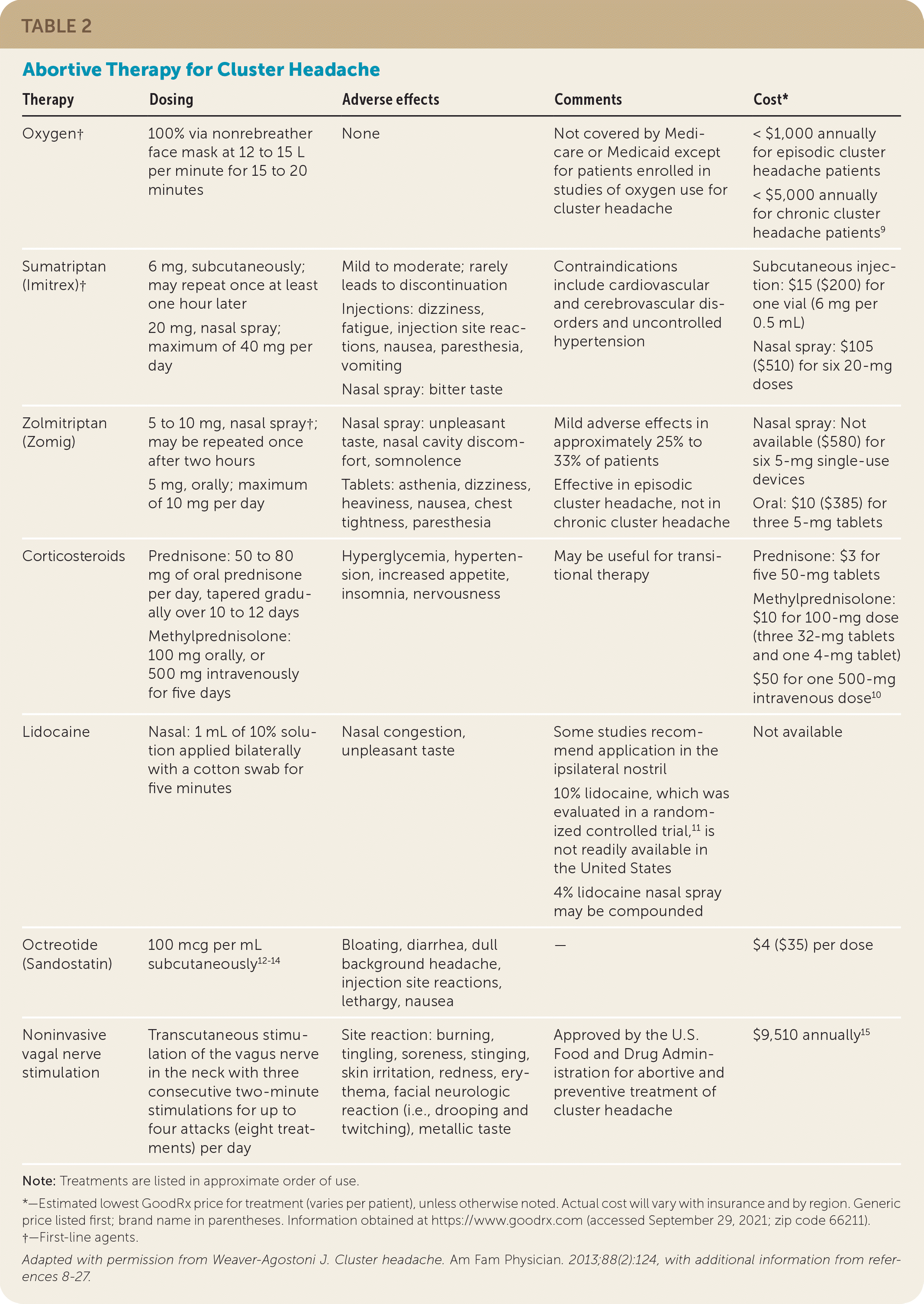
| Therapy | Dosing | Adverse effects | Comments | Cost* |
|---|---|---|---|---|
| Oxygen† | 100% via nonrebreather face mask at 12 to 15 L per minute for 15 to 20 minutes | None | Not covered by Medicare or Medicaid except for patients enrolled in studies of oxygen use for cluster headache | < $1,000 annually for episodic cluster headache patients < $5,000 annually for chronic cluster headache patients9 |
| Sumatriptan (Imitrex)† | 6 mg, subcutaneously; may repeat once at least one hour later 20 mg, nasal spray; maximum of 40 mg per day |
Mild to moderate; rarely leads to discontinuation Injections: dizziness, fatigue, injection site reactions, nausea, paresthesia, vomiting Nasal spray: bitter taste |
Contraindications include cardiovascular and cerebrovascular disorders and uncontrolled hypertension | Subcutaneous injection: $15 ($200) for one vial (6 mg per 0.5 mL) Nasal spray: $105 ($510) for six 20-mg doses |
| Zolmitriptan (Zomig) | 5 to 10 mg, nasal spray†; may be repeated once after two hours 5 mg, orally; maximum of 10 mg per day |
Nasal spray: unpleasant taste, nasal cavity discomfort, somnolence Tablets: asthenia, dizziness, heaviness, nausea, chest tightness, paresthesia |
Mild adverse effects in approximately 25% to 33% of patients Effective in episodic cluster headache, not in chronic cluster headache |
Nasal spray: Not available ($580) for six 5-mg single-use devices Oral: $10 ($385) for three 5-mg tablets |
| Corticosteroids | Prednisone: 50 to 80 mg of oral prednisone per day, tapered gradually over 10 to 12 days Methylprednisolone: 100 mg orally, or 500 mg intravenously for five days |
Hyperglycemia, hypertension, increased appetite, insomnia, nervousness | May be useful for transitional therapy | Prednisone: $3 for five 50-mg tablets Methylprednisolone: $10 for 100-mg dose (three 32-mg tablets and one 4-mg tablet) $50 for one 500-mg intravenous dose10 |
| Lidocaine | Nasal: 1 mL of 10% solution applied bilaterally with a cotton swab for five minutes | Nasal congestion, unpleasant taste | Some studies recommend application in the ipsilateral nostril 10% lidocaine, which was evaluated in a randomized controlled trial,11 is not readily available in the United States 4% lidocaine nasal spray may be compounded |
Not available |
| Octreotide (Sandostatin) | 100 mcg per mL subcutaneously12–14 | Bloating, diarrhea, dull background headache, injection site reactions, lethargy, nausea | — | $4 ($35) per dose |
| Noninvasive vagal nerve stimulation | Transcutaneous stimulation of the vagus nerve in the neck with three consecutive two-minute stimulations for up to four attacks (eight treatments) per day | Site reaction: burning, tingling, soreness, stinging, skin irritation, redness, erythema, facial neurologic reaction (i.e., drooping and twitching), metallic taste | Approved by the U.S. Food and Drug Administration for abortive and preventive treatment of cluster headache | $9,510 annually15 |
First-line therapies include triptan drugs and 100% oxygen.19
Subcutaneous sumatriptan (Imitrex), 6 mg, is a first-line treatment option. In two randomized placebo-controlled trials, sumatriptan provided relief as early as 15 minutes after being administered (74% decrease in headache severity in the sumatriptan group vs. 26% in the placebo group).2,8,20–22
Sumatriptan nasal spray, 20 mg, is considered a first-line option for acute bouts of cluster headache, although it acts slower than subcutaneous sumatriptan.28
Oral zolmitriptan (Zomig) is considered a second-line treatment option. It is effective only for acute attacks in episodic cluster headache.2,8,23
Zolmitriptan nasal spray, 5 mg or 10 mg, reduces headache severity at 30 minutes compared with placebo (number needed to treat [NNT] = 2 to 3 for 10 mg; NNT = 5 to 6 for 5 mg) and is considered a first-line therapeutic option.2,24,25
Adverse effects of triptans include paresthesia; heaviness felt in limbs, chest, head or neck, and other parts of the body; asthenia; nausea; and dizziness.8,23
Triptans are contraindicated in patients with cardiovascular disease, cerebrovascular disease, and uncontrolled hypertension.12
100% oxygen is considered first-line therapy for acute cluster headache. One randomized controlled trial found it effective at a flow rate of 12 L per minute for 15 minutes (NNT = 2).16,26
The U.S. Centers for Medicare and Medicaid Services does not cover home oxygen for cluster headache, but many private health care insurance providers reimburse the cost of oxygen use for this indication.9,27
Octreotide (Sandostatin), 100 mcg per mL administered subcutaneously, was shown to be superior to placebo (52% decrease in headache severity in the treatment group vs. 36% in the placebo group; NNT = 7) in one double-blind placebo-controlled crossover study. Gastrointestinal upset was the most common adverse effect. Octreotide is not contraindicated in patients with ischemic heart disease.13
Nasal lidocaine may be considered for treatment of acute cluster headache.8,11 In a small randomized crossover trial with 15 patients, 1 mL of 10% lidocaine administered nasally via a cotton swab to the area corresponding to the sphenopalatine fossa resulted in a shorter time to headache extinction compared with saline application (37 vs. 59 minutes; P < .05).11
Although intramuscular ergotamine, dihydroergotamine nasal spray, intravenous somatostatin receptor agonists, and oral prednisone have been shown to improve cluster headache response, one systematic review and meta-analysis concluded that there is insufficient evidence to recommend their use because of methodologic limitations of the studies.8 In a double-blind placebo-controlled trial, dihydroergotamine nasal spray reduced cluster headache severity compared with placebo (29.9% vs. 9%; NNT = 5).29
TRANSITIONAL THERAPY
Transitional therapy, such as suboccipital steroid injections and oral or intravenous corticosteroids, should be considered during initiation of prophylactic therapy to provide time for the prophylactic therapy to take full effect.12,14,30,31
First-line transitional therapy is suboccipital steroid injection.32,33
Only short-term use of corticosteroids is recommended because of serious adverse effects associated with their use.
PROPHYLACTIC THERAPY
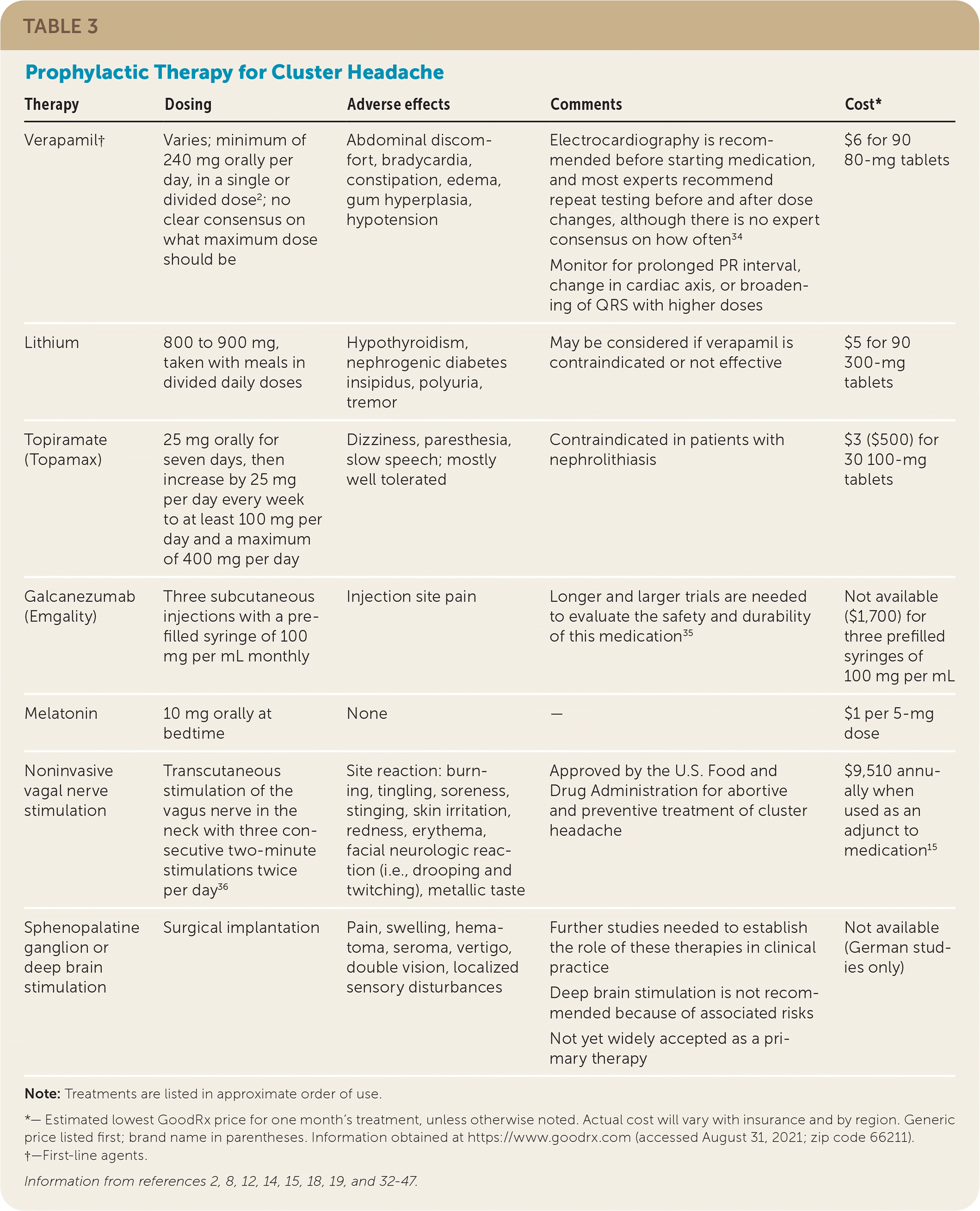
| Therapy | Dosing | Adverse effects | Comments | Cost* |
|---|---|---|---|---|
| Verapamil† | Varies; minimum of 240 mg orally per day, in a single or divided dose2; no clear consensus on what maximum dose should be | Abdominal discomfort, bradycardia, constipation, edema, gum hyperplasia, hypotension | Electrocardiography is recommended before starting medication, and most experts recommend repeat testing before and after dose changes, although there is no expert consensus on how often34 Monitor for prolonged PR interval, change in cardiac axis, or broadening of QRS with higher doses |
$6 for 90 80-mg tablets |
| Lithium | 800 to 900 mg, taken with meals in divided daily doses | Hypothyroidism, nephrogenic diabetes insipidus, polyuria, tremor | May be considered if verapamil is contraindicated or not effective | $5 for 90 300-mg tablets |
| Topiramate (Topamax) | 25 mg orally for seven days, then increase by 25 mg per day every week to at least 100 mg per day and a maximum of 400 mg per day | Dizziness, paresthesia, slow speech; mostly well tolerated | Contraindicated in patients with nephrolithiasis | $3 ($500) for 30 100-mg tablets |
| Galcanezumab (Emgality) | Three subcutaneous injections with a prefilled syringe of 100 mg per mL monthly | Injection site pain | Longer and larger trials are needed to evaluate the safety and durability of this medication35 | Not available ($1,700) for three prefilled syringes of 100 mg per mL |
| Melatonin | 10 mg orally at bedtime | None | — | $1 per 5-mg dose |
| Noninvasive vagal nerve stimulation | Transcutaneous stimulation of the vagus nerve in the neck with three consecutive two-minute stimulations twice per day36 | Site reaction: burning, tingling, soreness, stinging, skin irritation, redness, erythema, facial neurologic reaction (i.e., drooping and twitching), metallic taste | Approved by the U.S. Food and Drug Administration for abortive and preventive treatment of cluster headache | $9,510 annually when used as an adjunct to medication15 |
| Sphenopalatine ganglion or deep brain stimulation | Surgical implantation | Pain, swelling, hematoma, seroma, vertigo, double vision, localized sensory disturbances | Further studies needed to establish the role of these therapies in clinical practice Deep brain stimulation is not recommended because of associated risks Not yet widely accepted as a primary therapy |
Not available (German studies only) |
Verapamil is considered the first-line agent for cluster headache prophylaxis.2,12 A double-blind, parallel-group, randomized controlled trial comparing verapamil, 120 mg three times per day, with placebo found that those given verapamil were more likely to have a decrease of headache frequency by at least one-half (80% vs. 0%).37 Most experts agree that electrocardiography should be performed before beginning verapamil therapy, but there is no consensus on whether monitoring with electrocardiography should be done with dose changes.34
Galcanezumab (Emgality) is a monoclonal antibody targeting calcitonin gene–related peptide and is approved by the U.S. Food and Drug Administration for treatment of migraine. It was shown in a randomized trial to decrease headache frequency by at least one-half more often than with placebo during weeks 1 through 3 (71% vs. 53%; P < .05; NNT = 5).35 In that study, it was administered subcutaneously once per month as three consecutive injections with a pre-filled syringe of 100 mg per mL. The long-term safety and durability of the drug's benefit require longer studies.14
Nasal civamide, 50 mcg, may be modestly effective as prophylaxis for episodic cluster headache, but it is not available in the United States.18,38
Melatonin, 10 mg per day, may be considered for prevention of cluster headache, particularly in patients who are not able to tolerate other medications.8,19,39,40
Lithium may be considered if verapamil is contraindicated or not effective.12,42
Although topiramate (Topamax) has been recommended, there is no evidence from clinical trials.12,14
Valproate sodium does not appear to be an effective treatment option for cluster headache.8,14,39,43
SURGICAL TREATMENT
There are several invasive neurostimulation devices intended for the treatment of cluster headache.
In two randomized controlled trials, sphenopalatine ganglion stimulation decreased the frequency of chronic cluster headache attacks and provided acute relief.18,44 In one study, 67.1% of patients who received treatment with sphenopalatine ganglion stimulation achieved pain relief in 15 minutes compared with 7.4% in the sham treatment group (NNT = 2; P < .0001).44 Further research is needed to establish its role in clinical practice.18
There is insufficient evidence of benefit to recommend deep brain stimulation.45,46
Gamma Knife radiotherapy was studied in a prospective, nonblinded, open study, but the morbidity was too great for the low rate of pain cessation even in patients with refractory chronic cluster headache.48 In a more recent case series, Gamma Knife radiotherapy decreased cluster headache pain significantly in 60% of treated patients at 34 months' follow-up, but 50% of patients who were irradiated at the trigeminal nerve experienced facial sensory dysfunction.49
COMPLEMENTARY MEDICINE
Tryptamines, primarily psilocybin and lysergic acid diethylamide (LSD), have been reported on social forums to provide abortive and preventive therapy.50 Use of these substances is anecdotal, and no clinical trials of these drugs have been completed.
The effectiveness of cannabis for the treatment of cluster headache is unclear. Cannabis users have reported full symptom relief, some improvement, and exacerbation of symptoms.50
No alternative therapies were found to be consistently effective, including acupuncture, herbal treatment, chiropractic treatment, homeopathy, reflexology, hypnosis, spiritual therapy, massage, aromatherapy, relaxation therapy, physiotherapy, and yoga.51
OTHER TREATMENTS
The U.S. Food and Drug Administration has approved noninvasive vagus nerve stimulation for the treatment and prevention of acute episodic and chronic cluster headache. Two randomized controlled trials comparing noninvasive vagus nerve stimulation with sham stimulation found that headache response was significantly improved with nerve stimulation in patients with episodic cluster headache. There was no benefit in patients with chronic cluster headache.52,53 A study comparing standard of care alone with standard of care plus noninvasive vagus nerve stimulation found that nerve stimulation led to a significant, quick, sustained decrease in cluster headache frequency two weeks after it was added to standard of care.54
Prognosis
Longitudinal data on cluster headache are anecdotal and suggest that the natural history of the condition is to remit with age.51,55,56 However, in a study of 189 patients with cluster headache who were interviewed during a 10-year period, all patients continued to have cluster headache at the end of the study, although for some their chronic cluster headache evolved into episodic cluster headache and vice versa.57
This article updates previous articles on this topic by Weaver-Agostoni2 and Beck, et al.58
Data Sources: We searched Essential Evidence Plus, PubMed, and Google Scholar. Key words included cluster headache, cluster headache and causes, cluster headache and diagnosis, cluster headache treatment. The search included meta-analyses, randomized controlled trials, and medical society guidelines. Level of evidence and recommendations were derived from the American Headache Society's evidence-based guidelines. The European Federation of Neurologic Societies guidelines were also referenced. Search dates: November 2020 to February 2021 and September 2021.
The opinions and assertions contained herein are those of the authors and are not to be construed as official or as reflecting the views of the Uniformed Services University of the Health Sciences, the U.S. Air Force Medical Department, the Air Force at large, the U.S. Army Medical Department, the Army at large, or the U.S. Department of Defense.
