
Am Fam Physician. 2022;105(3):313-314
Author disclosure: No relevant financial relationships.
The i-STAT TBI Plasma test measures two proteins, ubiquitin C-terminal hydrolase-L1 (UCH-L1) and glial fibrillary acidic protein (GFAP). It has been approved by the U.S. Food and Drug Administration (FDA) for the detection of intracranial injury following mild traumatic brain injury (TBI). The test requires the use of the Abbott i-STAT TBI Plasma cartridge and the Abbott i-STAT Alinity analyzer to provide results.
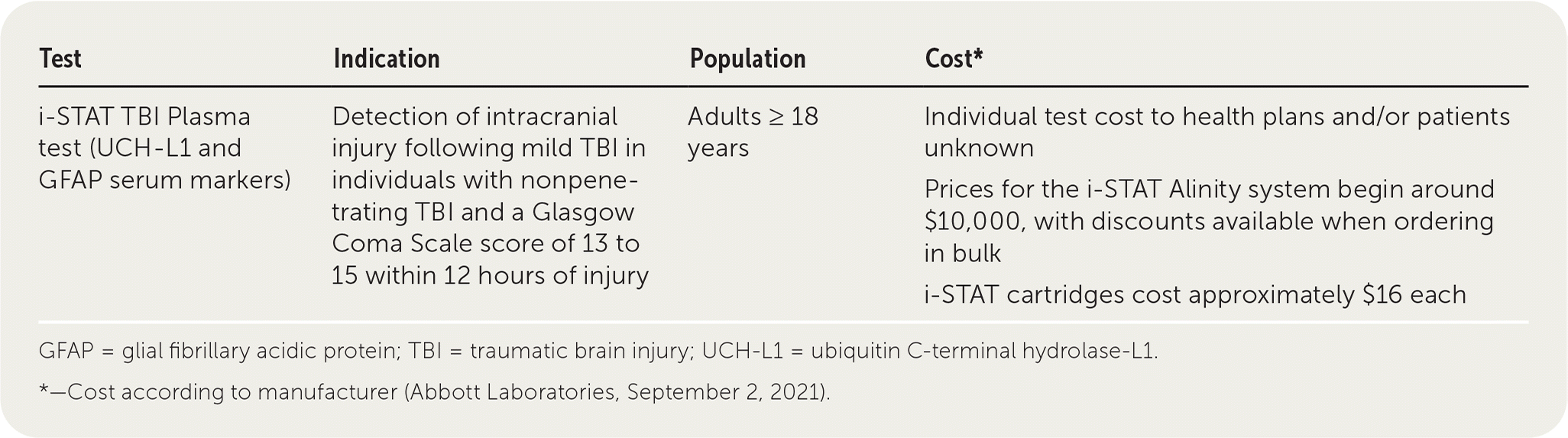
| Test | Indication | Population | Cost* |
|---|---|---|---|
| i-STAT TBI Plasma test (UCH-L1 and GFAP serum markers) | Detection of intracranial injury following mild TBI in individuals with nonpenetrating TBI and a Glasgow Coma Scale score of 13 to 15 within 12 hours of injury | Adults ≥ 18 years | Individual test cost to health plans and/or patients unknown Prices for the i-STAT Alinity system begin around $10,000, with discounts available when ordering in bulk i-STAT cartridges cost approximately $16 each |
Accuracy
A prospective, multicenter trial evaluated the accuracy of UCH-L1 and GFAP biomarkers in 1,959 adults who presented to an emergency department with mild TBI.1 Eligible participants had nonpenetrating TBI, had a Glasgow Coma Scale score between 9 and 15, and received head computed tomography (CT) and blood testing within 12 hours of injury. Using prespecified cutoff values of 327 pg per mL for UCH-L1 and 22 pg per mL for GFAP (for a negative result, both UCH-L1 and GFAP levels had to be below these values; for a positive result, one or both levels had to be above these values), the combined tests were 97.6% sensitive for intracranial head injuries compared with the reference standard of head CT. The negative predictive value was 99.6% for patients without intracranial injury on head CT who had a negative test result. The study authors calculated a positive likelihood ratio of 1.5 (95% CI, 1.455 to 1.616) and a negative likelihood ratio of 0.07 (95% CI, 0.00 to 0.153).
As part of its Breakthrough Devices Program, the FDA took only six months to approve UCH-L1 and GFAP testing as the Banyan Brain Trauma Indicator for the identification of intracranial injury following mild TBI.2 Preliminary studies of the accuracy of UCH-L1 and GFAP found that testing for both biomarkers was more predictive of intracranial bleeding than either alone.3,4 The i-STAT TBI Plasma test was approved by the FDA in January 2021 for use in patients with nonpenetrating TBI and a Glasgow Coma Scale score between 13 and 15 within 12 hours of injury.5
Benefit
More than 1 million individuals in the United States presented to the emergency department for TBI in 2017, the most recent year for which data are available.6 Many of these individuals received head CT to rule out intracranial injury; most of the results were negative.7 UCH-L1 and GFAP testing may avoid the need for CT. These serum tests can provide results in three to four hours, potentially reducing unnecessary radiation exposure and the cost of care.
Harms
UCH-L1 and GFAP testing has been validated only in patients with mild TBI clinically deemed to need head CT in an emergency department setting.3 Extrapolating these results to include patients with mild TBI who are initially not thought to need imaging, who present in a different care setting (e.g., primary care, urgent care, field of play), or who are outside of the 12-hour postinjury window may alter the sensitivity and specificity.
Cost
A 2019 cost-analysis determined that the Banyan Brain Trauma Indicator, an earlier iteration of UCH-L1 and GFAP testing used by the U.S. Department of Defense, would be cost-effective for avoiding unnecessary CT scans in mild TBI if the price were $309 or less.14
Abbott has not publicized the cost of the i-STAT TBI Plasma cartridge or the i-STAT Alinity analyzer. According to a manufacturer representative (Abbott Laboratories, September 2, 2021), the retail cost of the i-STAT Alinity system is approximately $10,000, with discounts available if purchasing multiple systems. Other i-STAT cartridges retail for approximately $16 per test.15
Bottom Line
UCH-L1 and GFAP are serum markers that may be used to reduce the need for head CT after mild TBI. However, there are no studies on the incorporation of these markers into existing head CT clinical decision rules or that describe the optimal use of the i-STAT TBI Plasma test outside of the emergency department setting.
Editor’s Note: Dr. Middleton is an assistant medical editor for AFP.
