Am Fam Physician. 2022;105(4):353-354
Published online March 1, 2022
Author disclosure: No relevant financial relationships.
The U.S. Food and Drug Administration
(FDA) recently approved the monoclonal antibody aducanumab (Aduhelm) for the treatment of early Alzheimer disease based solely on its ability to reduce amyloid in the brain.1 This commentary presents data to illuminate the controversy around this drug and provides guidance to physicians about how to approach newly approved drugs.
A comprehensive PubMed search (accessed date: November 2, 2021) identified two randomized trials reporting that aducanumab reduced amyloid deposition. A search of ClinicalTrials.gov (accessed date: November 2, 2021) identified 10 trials, including two that have been published. Four studies address bioavailability and dose-finding, one observational study and one trial have not begun recruitment, and four studies were terminated for futility. To our knowledge, no studies reporting clinical outcomes have been published, except in the FDA approval documents, which report the results of three otherwise unpublished randomized trials.1 These trials included 2,268 participants receiving placebo or aducanumab, 10 mg per kg intravenously every four weeks for six months to one year. All participants had mild cognitive impairment or mild Alzheimer disease and evidence of amyloid deposition on positron emission tomography. The studies lasted 54 to 78 weeks and were well-designed. They used validated instruments to assess clinical response: the Mini-Mental State Examination, the Clinical Dementia Rating-Sum of Boxes scale, and the Alzheimer’s Disease Assessment Scale-Cognitive Subscale.
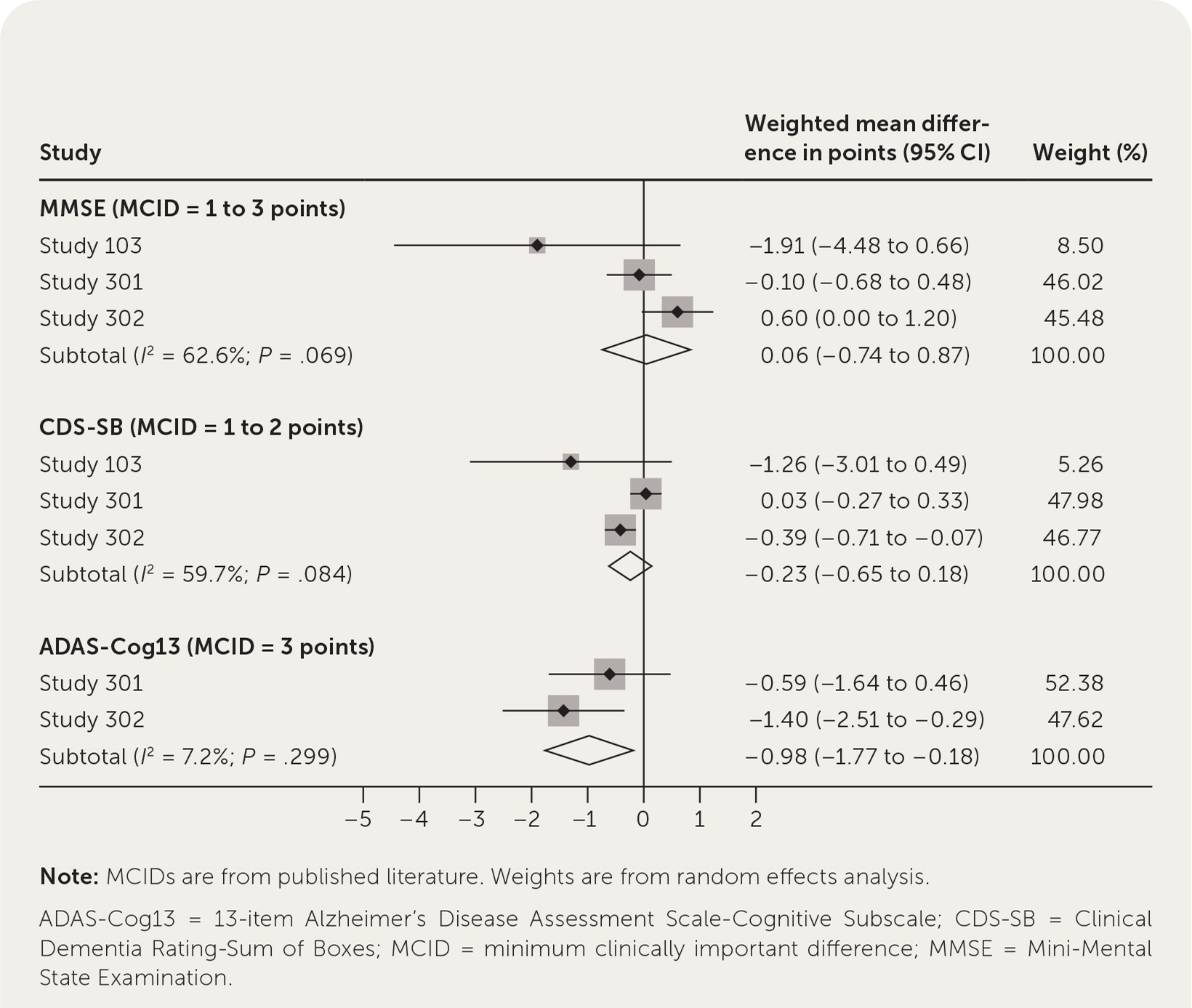
Because no meta-analysis has been published, we summarize our own in Figure 1. For all but one of the scales, we found published reports of the minimum clinically important difference (MCID) and used those in our analysis. The MCID is the minimum amount a scale would have to change for a patient or caregiver to notice improvement.2–4 Figure 1 shows that there were no statistically significant or clinically meaningful differences for the Mini-Mental State Examination and Clinical Dementia Rating-Sum of Boxes scores. Although the difference for the Alzheimer’s Disease Assessment Scale-Cognitive Subscale was statistically significant, it was not clinically important. An improvement of only 1 point was observed, whereas the MCID is at least 3 points; thus, the FDA approval was based entirely on amyloid reduction and ignored the absence of a significant symptomatic benefit for patients.
With regard to potential harms, aducanumab causes amyloid-related imaging abnormalities, including cerebral edema (35% of treated patients) and cerebral hemorrhage (21% of treated patients). In some patients, these changes were associated with headache (47%), confusion (15%), dizziness (11%), and nausea (8%).5 Although these abnormalities generally resolve with discontinuation of the medication, the FDA recommends performing brain magnetic resonance imaging at baseline and before the seventh and 12th infusions, with discontinuation of the medication if abnormalities are seen. Long-term safety is not known.
So, what can we glean from this? For more than two decades, family physicians have learned the importance of using clinically meaningful patient-oriented outcomes over surrogate markers.6 The available data suggest that aducanumab significantly decreases amyloid deposition but does not improve cognition in a clinically meaningful way. This is consistent with studies of other treatments for Alzheimer disease.7
Although the FDA approval process can be lengthy, the aducanumab manufacturer’s initial submission for approval was in July 2020, and it underwent an accelerated process that included an independent review by an advisory committee. Members of that committee voted 10 to one against approval, and three members resigned in protest after their guidance was overruled by FDA administrators.8,9 For a person weighing 163 lb (73.9 kg), the manufacturer initially estimated that aducanumab would cost $56,000 per year in the United States but recently reduced the price to $28,200. This does not include physician fees and the cost of multiple magnetic resonance imaging scans to monitor for amyloid-related imaging abnormalities.
Physicians should raise red flags when hearing about disagreements in the FDA approval process. Additionally, physicians need to demand that new drugs demonstrate clinically meaningful benefits to patients and that data regarding benefits, harms, and costs be reported in a manner that facilitates decision-making. One resource is the STEPS (Safety, Tolerability, Effectiveness, Price, and Simplicity) department in American Family Physician (https://www.aafp.org/afp/steps), which provides clear guidance on new drugs. According to the STEPS framework, new drugs should be safer, more tolerable, more effective, less costly, and simpler to use than current drugs. Aducanumab fails to meet almost every criteria: it has major safety concerns, it is not effective, it is costly, and it is complex to administer and monitor.
Although Alzheimer disease is a terrible and burdensome condition for patients and caregivers, approving an expensive drug that provides no meaningful benefit only creates false hope for these patients and caregivers. Given its high cost, we cannot afford to prescribe a “cure” that has been shown to improve only a biomarker (amyloid deposition on positron emission tomography) rather than a clinically meaningful patient-oriented outcome.
Editor’s note: Dr. Ebell is AFP’s deputy editor for evidence-based medicine.