
Am Fam Physician. 2022;106(1):91-92
Author disclosure: No relevant financial relationships.
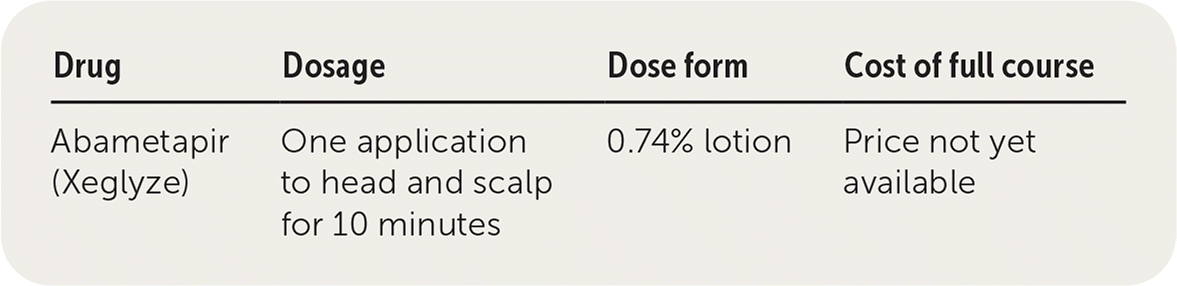
| Drug | Dosage | Dose form | Cost of full course |
|---|---|---|---|
| Abametapir (Xeglyze) | One application to head and scalp for 10 minutes | 0.74% lotion | Price not yet available |
Safety
No serious adverse effects were observed in patients receiving abametapir in premarketing trials. Safety has been established in patients six months and older.1,3 Abametapir is not recommended in patients younger than six months because of a potential risk of increased systemic absorption and adverse effects caused by the inactive ingredient, benzyl alcohol. No data are available on the use of abametapir in pregnant or breastfeeding patients.1
No contraindications are listed in the manufacturer labeling of abametapir. However, there is potential for inhibition of the cytochrome P450 (CYP) group of enzymes (CYP3A4, CYP3B6, CYP1A2) following a single application of abametapir, leading to possible increased systemic concentrations of medications processed by this enzyme system. The manufacturer recommends avoiding drugs metabolized by this group of enzymes within two weeks after application of abametapir. If avoidance is not possible, abametapir should not be used.1
Tolerability
Abametapir may cause local scalp reactions in a small number of patients (less than 5%). Contact with eyes should be avoided because irritation may occur.1
Effectiveness
In two randomized, double-blind, controlled studies involving a total of 704 patients older than six months, treatment with abametapir resulted in a greater likelihood of complete clearance of head lice at 14 days (85.9% in the treatment group vs. 61.3% in the placebo group; P < .001; number needed to treat = 4). Results were similar in children and adults.4
Ovicidal activity was demonstrated in a study of 50 patients. Eggs were collected before and after treatment with abametapir or vehicle. Compared with untreated eggs, the reduction in egg hatching was 92.9% for abametapir vs. 42.3% for vehicle (P < .001).2 Abametapir has not been compared with other nonprescription or prescription treatments of head lice, although older treatments may have clearance rates of less than 70% following two applications.5
Price
Although abametapir has been approved by the U.S. Food and Drug Administration, it is not yet available in pharmacies and prices are unknown. Nonprescription treatments such as permethrin lotion or pyrethrins/piperonyl butoxide shampoo will cost about $14 each for one bottle. Ivermectin 0.5% (Sklice) costs about $290 for one tube (117 g).
Simplicity
Treatment involves a single application of abametapir to coat dry hair and the scalp. Abametapir should be massaged into the scalp and hair and left on for 10 minutes before rinsing off with warm water. Contact with eyes should be avoided, and hands should be washed immediately after application. A fine-tooth or nit comb may be used to remove lice or nits. Patients may shampoo hair any time after treatment. Repeat treatment with abametapir is not necessary.1
Bottom Line
Abametapir is shown to be an effective treatment for head lice infestation in patients six months and older. It is safer than malathion and lindane and as effective as ivermectin and spinosad. However, it is expensive compared with permethrin or other nonprescription treatments. Abametapir may be considered an alternative when other over-the-counter and less expensive treatments are ineffective or cannot be used.
