Am Fam Physician. 2022;106(4):459-460
Author disclosure: No relevant financial relationships.
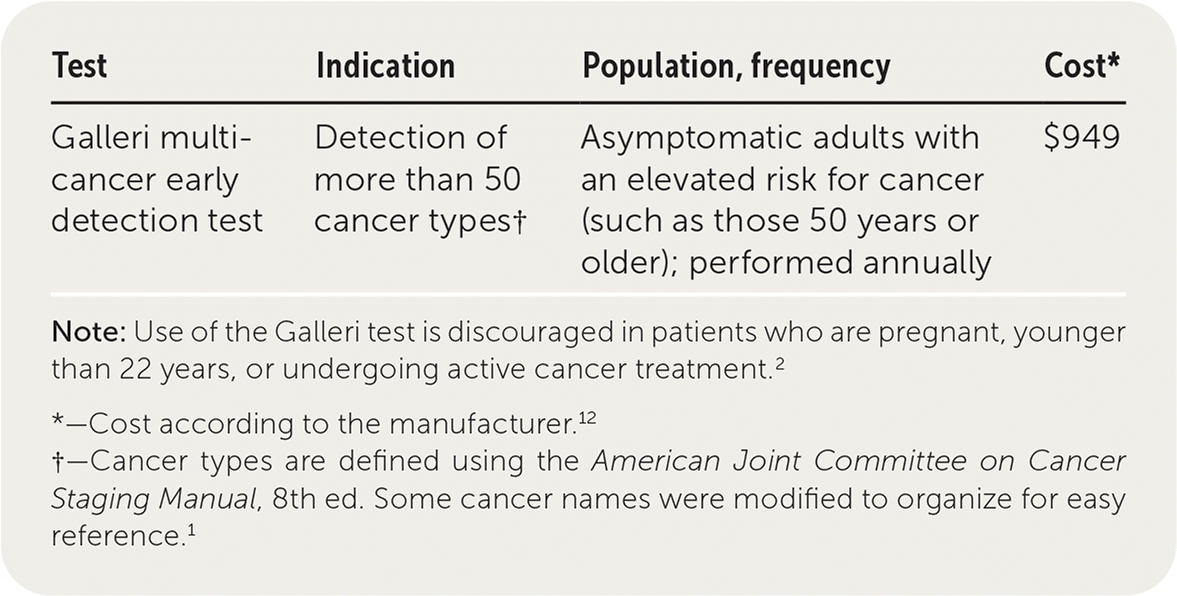
The Galleri test is a multicancer early detection blood test used to identify more than 50 cancer types in asymptomatic adults 50 years or older.1,2 It uses artificial intelligence analysis of cell-free DNA to recognize patterns associated with cancer and identify the likely tissue of origin. The Galleri test is not meant to replace current population-based cancer screening recommendations and is not approved by the U.S. Food and Drug Administration.3
Accuracy
An industry-funded prospective, multicenter, observational study characterized genomic cancer signals in blood samples of patients with and without cancer.4 The study enrolled 15,254 patients (56% with known cancer) and divided them into three substudies: discovery, training, and clinical validation. The discovery and training phases created a targeted methylation analysis of circulating cell-free DNA and assessed its performance in detecting and localizing multiple cancer types. Of note, comparing the test in patients who have known disease with healthy control patients will overestimate accuracy.
In the clinical validation phase, 2,823 patients with a cancer diagnosis and 1,254 patients without were assessed for cancer status after one year.4 The study did not report what proportion of patients with cancer were diagnosed using screening tests vs. those who presented with cancer symptoms. Specificity of the Galleri test for cancer signal detection was 99.5%. Overall sensitivity for cancer signal detection was 51.5% and increased with stage (I: 16.8%; II: 40.4%; III: 77.0%; IV: 90.1%). Sensitivity in stages I to III was 67.6% for the 12 prespecified cancer types that account for approximately two-thirds of annual U.S. cancer deaths and was 40.7% for all cancer types. Sensitivities for the 12 cancer types are shown in Table 1.4–6 Cancer signals were detected in more than 50 cancer types. The Galleri test correctly predicted cancer tissue of origin in 88.7% of true-positive cases.4
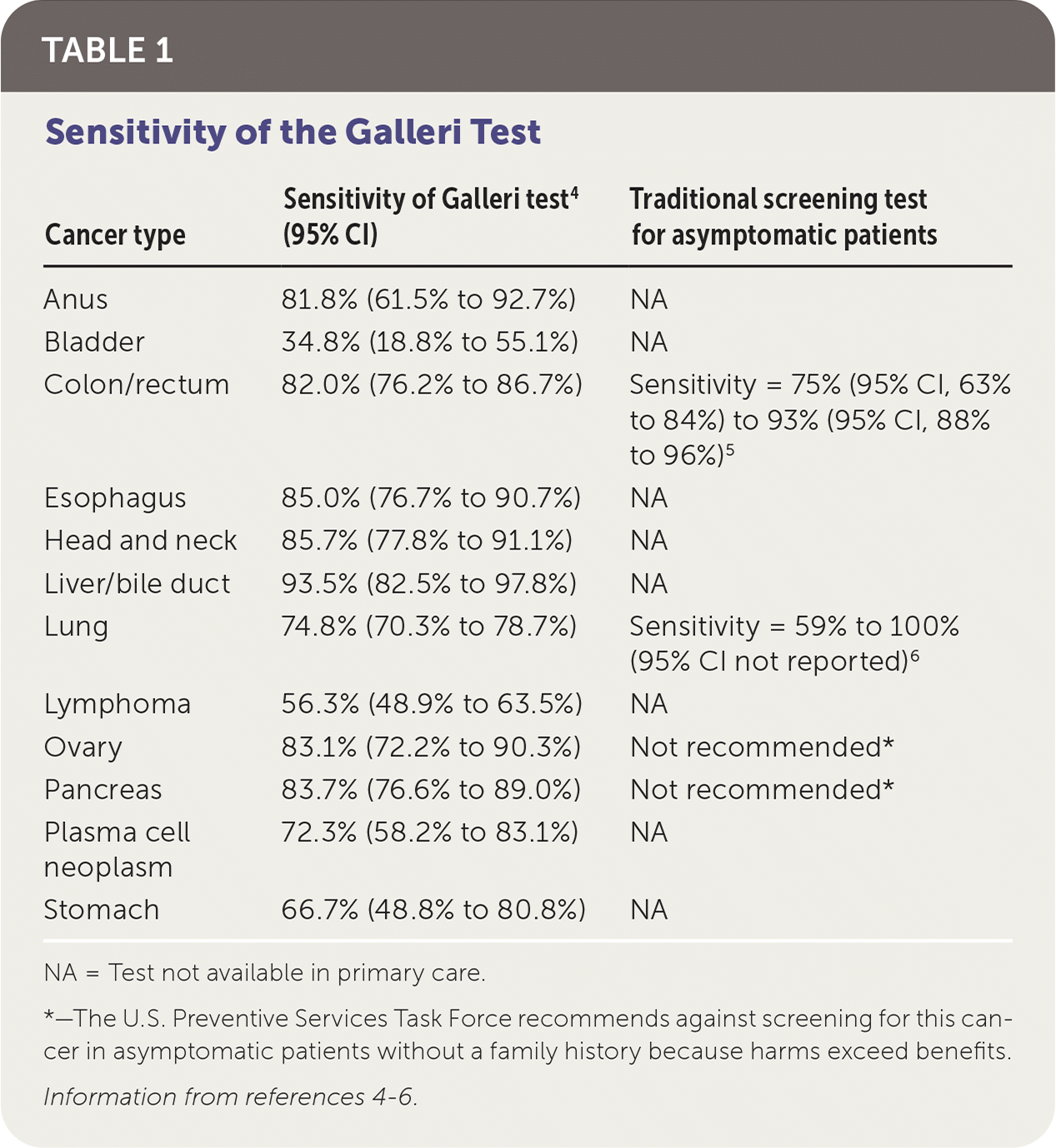
| Cancer type | Sensitivity of Galleri test4 (95% CI) | Traditional screening test for asymptomatic patients |
|---|---|---|
| Anus | 81.8% (61.5% to 92.7%) | NA |
| Bladder | 34.8% (18.8% to 55.1%) | NA |
| Colon/rectum | 82.0% (76.2% to 86.7%) | Sensitivity = 75% (95% CI, 63% to 84%) to 93% (95% CI, 88% to 96%)5 |
| Esophagus | 85.0% (76.7% to 90.7%) | NA |
| Head and neck | 85.7% (77.8% to 91.1%) | NA |
| Liver/bile duct | 93.5% (82.5% to 97.8%) | NA |
| Lung | 74.8% (70.3% to 78.7%) | Sensitivity = 59% to 100% (95% CI not reported)6 |
| Lymphoma | 56.3% (48.9% to 63.5%) | NA |
| Ovary | 83.1% (72.2% to 90.3%) | Not recommended* |
| Pancreas | 83.7% (76.6% to 89.0%) | Not recommended* |
| Plasma cell neoplasm | 72.3% (58.2% to 83.1%) | NA |
| Stomach | 66.7% (48.8% to 80.8%) | NA |
In a 2021 prospective interim analysis, 4,033 patients received Galleri testing, and clinical evaluation for cancer was performed if results were positive. A cancer signal was detected in 62 patients (1.5%). Of these patients, 40 reached diagnostic resolution; 18 had true-positive results and 22 had false-positive results after imaging and other diagnostic procedures (positive predictive value = 45%). Accuracy of the top cancer signal origin prediction in true-positive cases was 82.4%. Most cancers detected were diagnosed at stages I to III.7
Observational studies are ongoing for breast and lung cancers.8,9 Patients in these studies have provided blood samples, and traditional screening methods will be used to evaluate the performance of the Galleri test. A study in the United Kingdom is currently enrolling 140,000 National Health Service patients between 50 and 77 years of age to further evaluate test performance.10
Benefit
The Galleri test uses a blood draw and does not require a physician visit. This may be more convenient for patients living in rural or remote communities or who have other barriers to seeing a physician or accessing specialty or screening services. The Galleri test may help reduce racial and socioeconomic disparities in cancer screening adherence, but more research is needed.
Earlier diagnosis of cancer through Galleri testing has the potential to improve patient survival, although this has not been empirically demonstrated. Although most cancers are diagnosed in late stages, the Galleri test can screen for some cancers that do not have effective population-based screening options, which account for about 70% of cancer deaths in the United States.11
Harms
Because the Galleri test is not a diagnostic tool, a positive result must be confirmed in accordance with standard medical practice. Diagnostic decisions are the responsibility of the ordering physician. For some forms of cancer, there may be no effective treatment. When a cancer signal is detected, the likelihood that the individual has cancer remains elevated and may warrant further evaluation even after a negative diagnostic evaluation of the cancer signal origin(s). However, this process is not well defined and could lead to greater cost and harms.
False-positive or indeterminate results can cause patient anxiety. Indolent cancers may also be detected, leading to overdiagnosis and unnecessary treatments.
Cost
The Galleri test costs $949 and is not generally covered by insurance.12 The manufacturer reports that the test may be covered by some individual employers as an employee benefit.
Bottom Line
Preliminary data suggest that the Galleri test may complement established screening tests recommended by the U.S. Preventive Services Task Force. The out-of-pocket cost, potential need for further diagnostic evaluation, lower sensitivity at early stages of disease, and possible patient anxiety due to false-positive or indeterminate results must be considered before ordering this test. Widespread implementation or possible replacement of existing cancer screening tests will require additional long-term, prospective, population-based studies to further demonstrate accuracy, clinical utility, and improved patient-oriented outcomes.