Am Fam Physician. 2022;106(4):online
Author disclosure: No relevant financial relationships.

Details for This Review
Study Population: 288 patients with spontaneous bacterial peritonitis (SBP) from four randomized controlled trials
Efficacy End Points: Renal impairment and mortality
Harm End Points: Not reported
| Green | Benefits greater than harms |
| Yellow | Unclear benefits |
| Red | No benefits |
| Black | Harms greater than benefits |
Narrative: SBP is a potentially deadly complication of ascites in patients with cirrhosis, with mortality approaching 30%.1,2 Patients with cirrhosis and ascites are immunocompromised, and SBP can cause the release of proinflammatory mediators such as cytokines and nitric oxide within the bloodstream and ascitic fluid, which can lead to hypotension. Cirrhosis and ascites also result in splanchnic vasodilation, which can decrease the effective arterial volume and activate the sympathetic nervous system and renin-angiotensin-aldosterone system.2,3 This can contribute to renal impairment, which occurs in 30% to 40% of patients with SBP and is the strongest predictor of mortality in these patients.2–7
Intravenous albumin can restore effective circulating volume and may have anti-inflammatory properties.8–10 Although the American Association for the Study of Liver Diseases recommends albumin infusion in patients with SBP and renal or hepatic dysfunction, data on the routine use of albumin are unclear.11,12
A meta-analysis of four randomized controlled trials evaluated intravenous albumin infusion in 288 patients with SBP.13 The diagnosis of SBP was based on ascitic fluid polymorphonuclear cell counts of greater than 250 cells per mm3 in three trials and 250 cells per mm3 or greater in one trial. All patients received antibiotics. Albumin was compared with no albumin (three trials) or artificial colloid (one trial). All trials used albumin 20% solution, with a dose of 0.5 to 1.5 g per kg in three trials and three daily fixed doses of 10 g in one trial. Albumin infusion was continued for three days in three trials and three weeks in one trial. In all trials, exclusion criteria were antibiotic use in the preceding week, existing cardiac disease, HIV or other unspecified infection, grade 3 or 4 liver encephalopathy, gastrointestinal bleeding, and advanced age. Studies comparing different doses of albumin or evaluating albumin infusion associated with paracentesis were excluded from the meta-analysis.
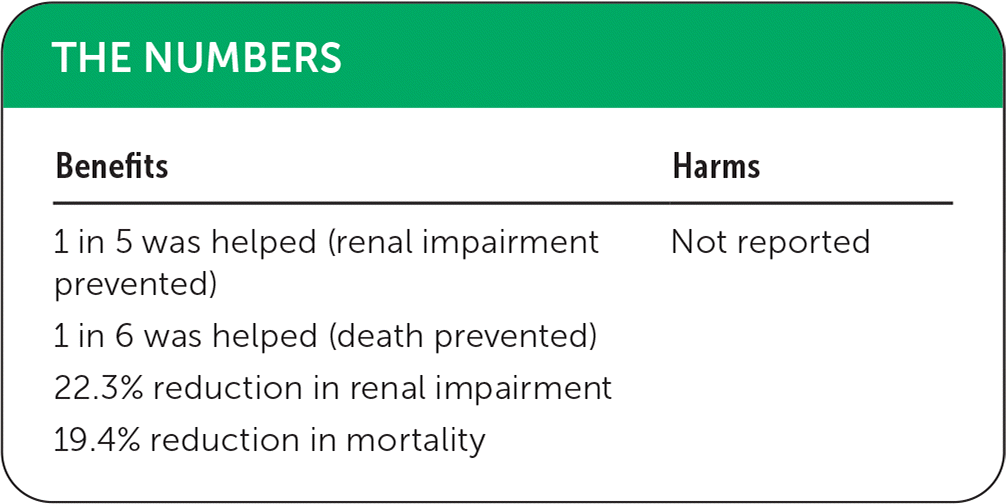
Outcomes of the meta-analysis included renal impairment, which was defined in each trial (e.g., preexisting renal insufficiency, increases in serum creatinine or blood urea nitrogen), and in-hospital mortality. Intravenous albumin infusion was associated with reduced renal impairment (odds ratio [OR] = 0.21; 95% CI, 0.11 to 0.42; absolute risk reduction [ARR] = 22.3%; number needed to treat [NNT] = 5) and in-hospital mortality (OR = 0.34; 95% CI, 0.19 to 0.60; ARR = 19.4%; NNT = 6). None of the trials reported on the harms associated with albumin infusion.
Caveats: The meta-analysis had several limitations. Although the results suggest that albumin infusion decreases renal impairment and mortality in patients with SBP, only four trials were included, and 238 of the 288 patients were from two trials.10,14 The quality of included studies was poor to fair. Only one trial was blinded,10 and one trial used a coin flip for randomization (i.e., pseudorandomization).14 There were also several sources of heterogeneity. Antibiotic type and dosing differed among trials, although all were accepted SBP regimens. All studies used albumin 20% infusion, but dosing regimens and duration varied. The length of follow-up was unspecified in three trials; one trial followed patients for 90 days. The meta-analysis was funded by a company that makes albumin.
Conclusion: This meta-analysis found that albumin infusion decreases the risk of renal impairment and mortality in patients with SBP and no demonstrated renal or hepatic impairment. Based on this evidence, we have assigned a color recommendation of green (benefits greater than harms) for the use of albumin infusion in these patients. However, the low quality of evidence and lack of reporting of harms warrant caution in interpreting the results. Further high-quality data are needed to confirm the benefits and evaluate the harms of this intervention.