
Am Fam Physician. 2023;107(2):134-135
Author disclosure: No relevant financial relationships.
Clinical Question
Are fecal immunochemical tests (FITs) superior to guaiac-based fecal occult blood tests (gFOBTs) when screening individuals at average risk for colorectal cancer?
Evidence-Based Answer
FITs are more likely than gFOBTs to detect colorectal cancer or advanced adenomas in individuals at average risk.1 (Strength of Recommendation = A, consistent, good-quality patient-oriented evidence.)
Practice Pointers
In 2019, colorectal cancer was the fourth most common cancer in the United States and was the fourth highest cause of cancer-related mortality.2 Screening with a gFOBT or FIT can find colorectal cancer during the presymptomatic phase or detect advanced adenomas, potential precursors to colorectal cancer. Early identification leads to easier treatments and lower mortality.1 The authors of the review sought to compare the accuracy of gFOBTs vs. FITs for population-based screening for colorectal cancer and advanced adenomas in individuals at average risk.
The Cochrane review included 63 high-quality studies and more than 3.7 million patients older than 40 years.1 The included studies used a variety of methodologies, controls, and comparisons, which led the authors to analyze them using two primary constructs. Studies in which participants received both a fecal test and a colonoscopy were termed reference standard: all. Studies that used a sequential screening method in which only a positive fecal screening was followed by colonoscopy were termed reference standard: positive. The authors only included studies that used colonoscopy as the reference standard.
In the reference standard: all analysis (n = 126,378), FIT screening was more sensitive at detecting colorectal cancer and advanced adenomas compared with gFOBTs. There was no difference in specificity, meaning gFOBTs and FITs had similar false-positive rates. The reference standard: positive analysis (n = 2,624,005) found FITs to be superior to gFOBTs at accurately identifying patients with colorectal cancer. In this analysis, FITs resulted in more follow-up colonoscopy than gFOBTs, with a specificity of 94% vs. 98%, respectively. Although FITs demonstrated superior sensitivity, it is important to recognize that sensitivity rates for both screening tests are low, particularly for the detection of advanced neoplasia. Table 1 summarizes the findings of these meta-analyses.
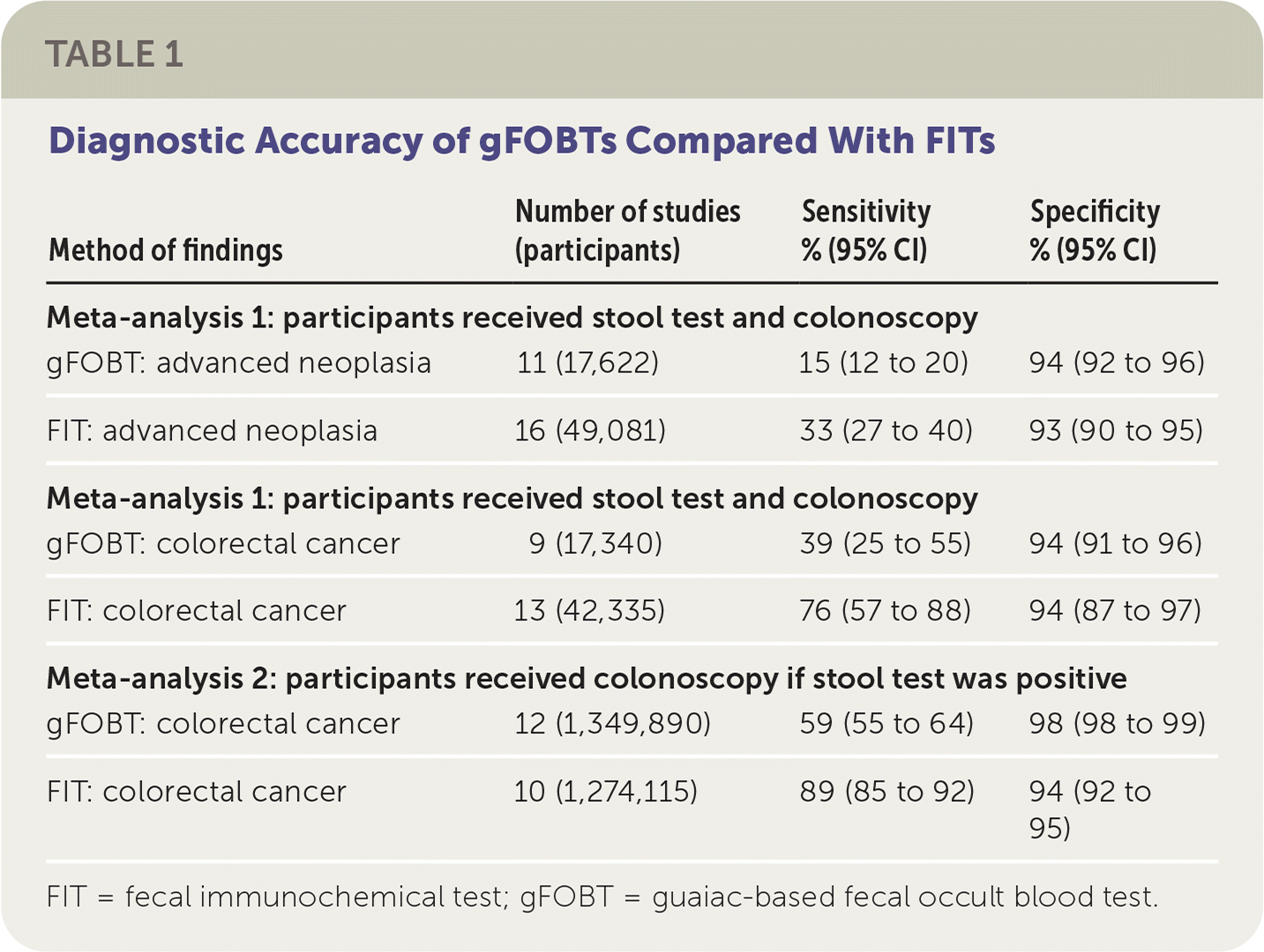
| Method of findings | Number of studies (participants) | Sensitivity % (95% CI) | Specificity % (95% CI) |
|---|---|---|---|
| Meta-analysis 1: participants received stool test and colonoscopy | |||
| gFOBT: advanced neoplasia | 11 (17,622) | 15 (12 to 20) | 94 (92 to 96) |
| FIT: advanced neoplasia | 16 (49,081) | 33 (27 to 40) | 93 (90 to 95) |
| Meta-analysis 1: participants received stool test and colonoscopy | |||
| gFOBT: colorectal cancer | 9 (17,340) | 39 (25 to 55) | 94 (91 to 96) |
| FIT: colorectal cancer | 13 (42,335) | 76 (57 to 88) | 94 (87 to 97) |
| Meta-analysis 2: participants received colonoscopy if stool test was positive | |||
| gFOBT: colorectal cancer | 12 (1,349,890) | 59 (55 to 64) | 98 (98 to 99) |
| FIT: colorectal cancer | 10 (1,274,115) | 89 (85 to 92) | 94 (92 to 95) |
Additional characteristics of FOBTs are relevant in the overall context of cancer screening. Ideal gFOBT screening requires the collection of two fecal samples from three consecutive bowel movements, whereas FIT screening requires only one sample. FIT screening can provide quantitative results that allow for adjusting cut-off values or potentially triaging positive results so that patients with more bleeding are able to get a colonoscopy earlier. FIT screening does not require any alteration in diet before testing.
Colon cancer screening is recommended for all adults in the United States, beginning at 50 years of age; some guidelines recommend starting at 45 years.3,4 FITs and gFOBTs are among the recommended options for colorectal cancer screening. Alternative methods of screening, including colonoscopy, computed tomography colonography, and stool DNA-FITs, were not considered in this direct comparison of FITs vs. gFOBTs. Positive screening tests for fecal occult blood must be followed by colonoscopy regardless of the stool test chosen. Patient preference, life expectancy, health system resources, local costs, and logistics should be considered when making colon cancer screening decisions.
The practice recommendations in this activity are available at https://www.cochrane.org/CD009276.
The opinions or assertions contained herein are the private views of the authors and are not to be construed as official or as reflecting the views of the U.S. Department of Defense or the Uniformed Services University of the Health Sciences.
