
Am Fam Physician. 2023;107(5):466-473
Related Letter to the Editor: Allergic Rhinitis: Rapid Evidence Review
Patient information: See related handout on controlling allergy symptoms.
Author disclosure: No relevant financial relationships.
Allergic rhinitis, the fifth most common chronic disease in the United States, is an immunoglobulin E–mediated process. A family history of allergic rhinitis, asthma, or atopic dermatitis increases a patient's risk of being diagnosed with allergic rhinitis. People in the United States are commonly sensitized to grass, dust mites, and ragweed allergens. Dust mite–proof mattress covers do not prevent allergic rhinitis in children two years and younger. Diagnosis is clinical and based on history, physical examination, and at least one symptom of nasal congestion, runny or itchy nose, or sneezing. History should include whether the symptoms are seasonal or perennial, symptom triggers, and severity. Common examination findings are clear rhinorrhea, pale nasal mucosa, swollen nasal turbinates, watery eye discharge, conjunctival swelling, and allergic shiners (i.e., dark circles under the eyes). Serum or skin testing for specific allergens should be performed when there is inadequate response to empiric treatment, if diagnosis is uncertain, or to guide initiation or titration of therapy. Intranasal corticosteroids are first-line treatment for allergic rhinitis. Second-line therapies include antihistamines and leukotriene receptor antagonists and neither shows superiority. If allergy testing is performed, trigger-directed immunotherapy can be effectively delivered subcutaneously or sublingually. High-efficiency particulate air (HEPA) filters are not effective at decreasing allergy symptoms. Approximately 1 in 10 patients with allergic rhinitis will develop asthma.
Allergic rhinitis is the most common chronic disease of childhood and the fifth most common chronic disease in the United States.1,2 It is an immunoglobulin E (IgE)–mediated process, and a clinical diagnosis is made based on common signs and symptoms, physical examination findings, and family and social history. This rapid evidence review highlights current literature and research on the diagnosis and treatment of allergic rhinitis.
Epidemiology and Pathophysiology
Approximately 15% to 30% of people in the United States have allergic rhinitis. Estimates of the direct economic burden range from $2 billion to $5 billion annually.2,3
Allergic rhinitis is caused by IgE-mediated reactions against inhaled allergens. IgE-mediated cross-linking activates mast cells and basophils, releasing histamine and leukotrienes that cause edema, vasodilation, nasal obstruction, and central nervous system reflexes that cause sneezing.1
In the United States, patients with allergic rhinitis are commonly sensitized to grass, dust mites, and ragweed allergens.4
Allergic rhinitis is the most common medical reason employees miss time from work and is associated with the largest productivity loss for employers.5
In children and adolescents, allergic rhinitis is associated with increased school absenteeism, irritability, inattention, and sleep disruption.2,3,6
Prevention
Diagnosis
HISTORY
The American Academy of Otolaryngology– Head and Neck Surgery Foundation's allergic rhinitis guideline was endorsed by the American Academy of Family Physicians in 2014 and reaffirmed in April 2020.2,9
Diagnosis of allergic rhinitis is based on patient history, physical examination, and at least one of the following symptoms: nasal congestion, runny or itchy nose, or sneezing.2 Table 1 shows the accuracy of medical history for the diagnosis of allergic rhinitis.10
Other symptoms include itchy or watery eyes, sniffling, and postnasal drip. History should include whether the symptoms are seasonal or perennial, symptom triggers, and severity. Family history of allergic rhinitis, asthma, or atopic dermatitis makes the diagnosis more likely.2,10,11
Triggers unlikely to be related to allergic rhinitis (e.g., smoke, fumes, chemicals), unilateral nasal symptoms, and the use of medications known to cause nasal symptoms (e.g., antihypertensives such as beta blockers, calcium channel blockers, and angiotensin-converting enzyme inhibitors; psychotropic agents; rebound effects from topical decongestants) suggest another possible diagnosis.12,13

| Question | Positive likelihood ratio | Negative likelihood ratio |
|---|---|---|
| Are allergic nasal symptoms from pollen or animals? | 6.7 | 0.15 |
| Are symptoms from animals? | 4.2 | 0.34 |
| Do any family members have asthma, eczema, or allergic rhinitis? | 3.4 | 0.70 |
| Do house dust, house dust mites, or pollen provoke or increase nasal symptoms? | 3.3 | 0.39 |
| Do you think you are allergic? | 3.1 | 0.33 |
| Are nasal symptoms accompanied by itchy watery eyes? | 2.5 | 0.51 |
| Do you have seasonal exacerbation? | 1.6 | 0.59 |
| Nasal symptoms in the past year, including sneezing, runny nose, or blocked nose, when you did not have a cold or flu? | 1.4 | 0.14 |
PHYSICAL EXAMINATION
Physical examination findings are often nonspecific but increase the likelihood of allergic rhinitis when combined with history.12
Common examination findings include clear rhinorrhea, pale nasal mucosa, swelling of the nasal turbinates, watery eye discharge, conjunctival swelling, and allergic shiners (i.e., dark circles under the eyes).12
Interrater reliability of the clinical assessment of turbinate hypertrophy (kappa = 0.31, range = 0 to 1.0) and turbinate color (kappa = 0.38) is modest.14
DIAGNOSTIC TESTING
It is reasonable to diagnose and begin empiric treatment for allergic rhinitis based on history and physical examination.2,9
Allergy testing should be performed when there is inadequate response to empiric treatment, when diagnosis is uncertain, or to guide initiation or titration of therapy.2
Serum and skin tests are the two main types of allergy testing15 (Table 213).
Blood serum tests determine the level of allergen-specific IgE in a serum sample.1 However, IgE levels do not necessarily correlate with clinical severity. In one study, the highest levels of IgE induced the weakest biologic reactions (i.e., mean wheal diameter of skin reaction).16
Skin testing is more sensitive (80% to 90%) compared with blood serum testing (average sensitivity = 70% to 75%), less expensive, and provides immediate results.17–20 Blood serum testing is more specific (80% to 100%).21
Blood testing should be used if there are contraindications to skin testing (e.g., high risk of anaphylaxis, severe dermatologic conditions) or if the patient prefers it to skin testing.2,9
Insufficient evidence exists to support other types of testing (e.g., nasal allergen challenges, acoustic rhinometry).12
Do not routinely perform sinonasal imaging unless there are other clinical indications (e.g., evidence of acute or chronic sinusitis, nasal polyps, suspicion for neoplasm).2,9
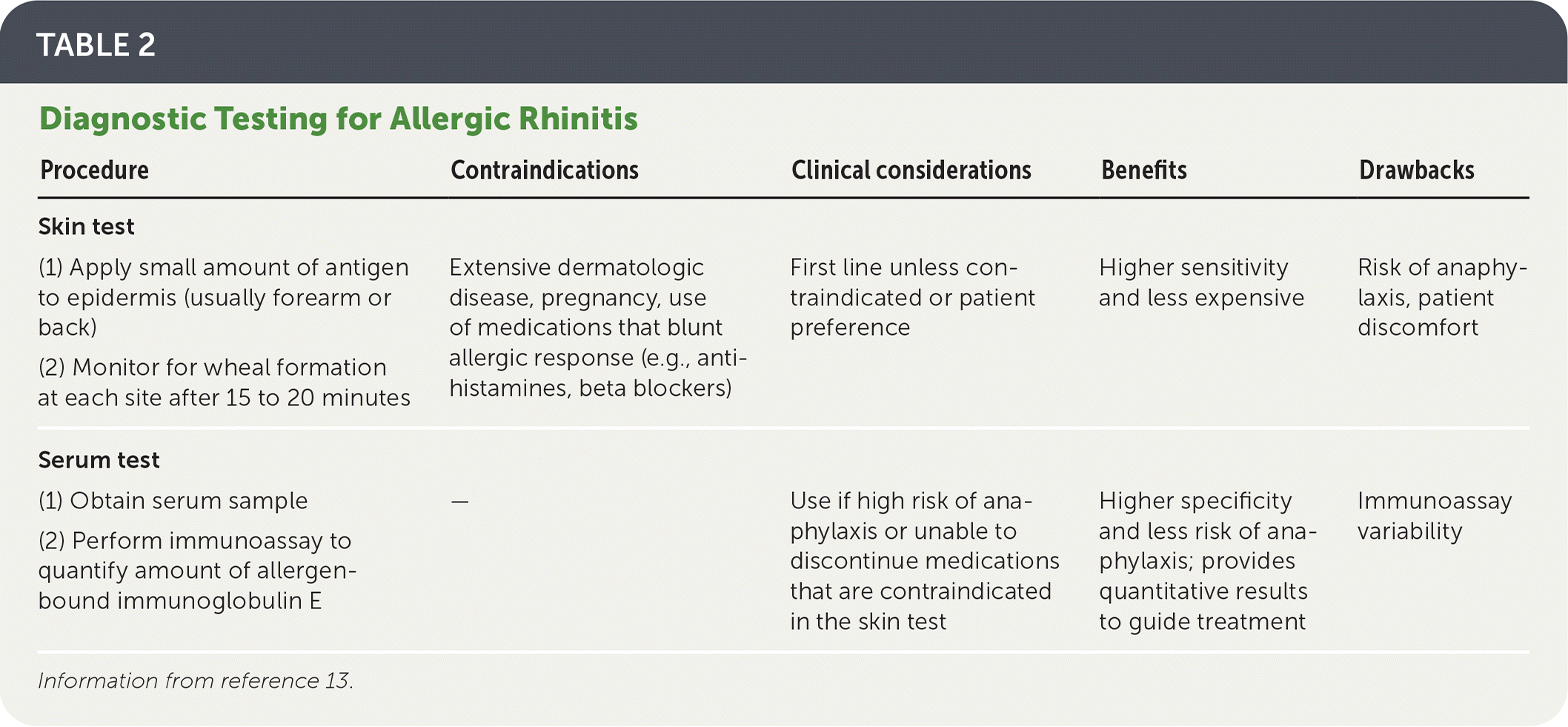
| Procedure | Contraindications | Clinical considerations | Benefits | Drawbacks |
|---|---|---|---|---|
| Skin test | ||||
| (1) Apply small amount of antigen to epidermis (usually forearm or back) (2) Monitor for wheal formation at each site after 15 to 20 minutes | Extensive dermatologic disease, pregnancy, use of medications that blunt allergic response (e.g., antihistamines, beta blockers) | First line unless contraindicated or patient preference | Higher sensitivity and less expensive | Risk of anaphylaxis, patient discomfort |
| Serum test | ||||
| (1) Obtain serum sample (2) Perform immunoassay to quantify amount of allergen-bound immunoglobulin E | — | Use if high risk of anaphylaxis or unable to discontinue medications that are contraindicated in the skin test | Higher specificity and less risk of anaphylaxis; provides quantitative results to guide treatment | Immunoassay variability |
Treatment
MEDICATION
Table 3 shows different treatment options for allergic rhinitis. Table 4 outlines the adverse effects of these medications.22–24
Intranasal corticosteroids are first-line treatment for allergic rhinitis, even when used on an as-needed basis, with clear superiority over other medical therapies. One randomized controlled trial (RCT) showed no significant difference in mean change of total nasal symptom score after six weeks between regular and intermittent use. Another randomized, open-label, parallel-group study comparing fluticasone with loratadine showed that the median total symptom score using an allergic rhinitis–specific scale was significantly lower, meaning fewer symptoms, in the fluticasone group compared with the loratadine group (4 vs. 7 points out of a total of 7).25–29
There are fewer studies supporting the use of intranasal corticosteroids for allergic rhinitis in children, and they are lower quality (three RCTs, 79 participants) than those in adults.30,31
Avoid using intranasal corticosteroids, particularly those metabolized via CYP3A4, such as fluticasone and budesonide, in patients with HIV who are being treated with ritonavir or cobicistat (Tybost). Medication interactions can increase steroid levels and risk of systemic corticosteroid effects, including Cushing syndrome and serious infection. Mometasone should be used cautiously with ritonavir because of pharmacokinetic similarities to fluticasone.32
One double-blind, parallel, three-group study showed that treating allergic rhinitis with intranasal corticosteroids improved control of asthma symptoms and decreased morbidity.33
Montelukast alone is ineffective to treat allergic rhinitis, but it may be considered as an adjunct therapy.34,35 A systematic review and meta-analysis concluded that montelukast did not improve nasal symptom scores in a clinically significant manner. Topical nasal corticosteroids were significantly more effective than montelukast (8.4% greater improvement in symptom scores; 95% CI, 6% to 11%). Combining montelukast with an antihistamine controlled symptoms better than either alone, and two studies showed similar clinical effectiveness to nasal corticosteroids. One RCT showed equivalent effectiveness with montelukast and loratadine, although neither was as effective as intranasal corticosteroids.34,35
Oral antihistamines are inferior to intranasal corticosteroids in relieving nasal symptoms (standardized mean difference [SMD] = −0.70; 95% CI, −0.93 to −0.47) and quality of life (SMD = −0.90; 95% CI, −1.18 to −0.62), but there is no significant difference for ocular symptoms.36 Studies on nasal antihistamines are lacking.
Normal saline nasal irrigation is more effective than saline spray in decreasing nasal or sinus symptoms, and low-quality evidence shows that it may decrease patient-reported disease severity with no adverse effects compared with no saline irrigation at three months in adults and children. There was an approximate decrease of 3 points on a 10-point visual analog scale for nasal symptoms.22,37
Omalizumab (Xolair), an anti-IgE antibody, effectively treats allergic rhinitis, but it is approved by the U.S. Food and Drug Administration only for allergic asthma, nasal polyps, and chronic idiopathic urticaria. It has not been studied compared with other treatment strategies.38
Biologics such as timothy grass pollen allergen extract (Grastek) and house dust mite allergen extract (Odactra) are costly and not recommended for uncomplicated allergic rhinitis. Sublingual immunotherapy, when indicated, is moderately effective for treating allergic rhinitis based on symptom scores (SMD = −0.49; 95% CI, −0.64 to −0.34). Grass pollen sublingual immunotherapy demonstrated more adverse events than placebo (61.3% vs. 20.9%).23,24 No head-to-head studies between subcutaneous and sublingual immunotherapy exist; choice depends on patient preference or availability.39
For most adults, oral sublingual treatment with grass pollen extract is not effective at decreasing symptoms related to grass pollen allergy.23 For a patient with an isolated grass pollen allergy, daily sublingual treatment with grass pollen extract is somewhat effective in reducing rhinoconjunctivitis.32
Oral and nasal decongestants lack rigorous studies for allergic rhinitis, but one low-quality study showed similar improvement in symptoms with pseudoephedrine and montelukast, although pseudoephedrine was marginally better for nasal congestion.40
Allergist referral should be considered if diagnosis is questionable, first- and second-line treatments are ineffective, or the patient is interested in pursuing immunotherapy.
| Type of therapy | Mechanism of action | Minimum age for use | Cost* | FDA pregnancy category |
|---|---|---|---|---|
| Intranasal corticosteroids | ||||
| Beclomethasone (Qnasl) | Inhibits multiple inflammatory cytokines | 4 years | — ($250) for one inhaler | May use during pregnancy; no human data but considered low risk |
| Budesonide | 6 years | $9 (—) for one nasal spray | ||
| Ciclesonide (Omnaris) | 6 years | — ($300) for one nasal spray | ||
| Flunisolide | 6 years | $25 (—) for one nasal spray | ||
| Fluticasone furoate (Flonase Sensimist) | 2 years | $5 ($20) for one nasal spray | ||
| Fluticasone propionate | 4 years | $5 (—) for one nasal spray | ||
| Mometasone | 2 years | $30 (—) for one nasal spray | ||
| Triamcinolone | 2 years | $21 (—) for one nasal spray | ||
| Oral antihistamines | ||||
| Cetirizine (Zyrtec) | First-generation antihistamines nonselectively antagonize central and peripheral histamine H1 receptors; second-generation antihistamines selectively antagonize peripheral H1 receptors | 6 months | $2 ($20) for 30 tablets | May use during pregnancy based on limited human data |
| Chlorpheniramine | 6 years | $1 (—) for 30 tablets | ||
| Desloratadine (Clarinex) | 6 months for perennial use and 2 years for seasonal use | $8 ($230) for 30 tablets | ||
| Diphenhydramine | 6 years | $4 (—) for 30 tablets | ||
| Fexofenadine | 12 years | $5 (—) for 30 tablets | ||
| Levocetirizine | 6 months for seasonal use and 6 years for perennial use | $5 (—) for 30 tablets | ||
| Loratadine | 2 years | $4 (—) for 30 tablets | ||
| Intranasal antihistamines | ||||
| Azelastine | Antagonizes central and peripheral H1 receptors (nonselective antihistamine) | 5 years for seasonal use and 6 years for perennial use | $15 (—) for one nasal spray | May use during pregnancy; no human data although fetal harm not expected based on limited systemic absorption (40%) |
| Combination intranasal corticosteroid and antihistamine | ||||
| Azelastine/fluticasone (Dymista) | Inhibits multiple inflammatory cytokines; antagonizes central and peripheral H1 receptors (nonselective antihistamine) | 6 years | $70 ($120) for one nasal spray | May use during pregnancy; no human data, although fetal harm not expected based on limited systemic absorption |
| Mometasone/olopatadine (Ryaltris) | 12 years | — ($250) for one nasal spray | ||
| Oral decongestants | ||||
| Pseudoephedrine | Stimulates smooth muscle alpha-adrenergic receptors, producing vasoconstriction and reducing nasal congestion (sympathomimetic) | 6 years | $4 (—) for 30 tablets | Avoid use in first trimester; weigh risks and benefits in second and third trimesters; limited human data suggest risk of teratogenicity including gastroschisis, small intestinal atresia, and hemifacial microsomia; risk of vasoconstriction based on animal data and mechanism of action |
| Intranasal cromolyns and anticholinergics | ||||
| Cromolyn | Inhibits mast cell degranulation (mast cell stabilizer) | 2 years | $7 (—) for one nasal spray | May use during pregnancy; no human data, although fetal harm not expected based on limited systemic absorption (< 7%) |
| Ipratropium | Antagonizes acetylcholine receptors, inhibiting nasal serous/seromucous gland secretions | 5 years for seasonal use and 6 years for perennial use | $14 (—) for one nasal spray | May use during pregnancy; no human data, although fetal harm not expected based on limited systemic absorption (< 20%) |
| Leukotriene receptor antagonists | ||||
| Montelukast | Selectively binds to cysteinyl leukotriene receptors, decreasing inflammation and swelling | 6 months for perennial use, 2 years for seasonal use | $15 (—) for 30 tablets | Weigh risks and benefits during pregnancy; no known risk based on limited human and animal data |
| Sublingual immunotherapy | ||||
| Timothy grass pollen allergen extract, cross-reactive with six other grass pollens (Grastek) | Exact mechanism unknown: alters immune response and promotes tolerance through repeated allergen-specific exposure | 5 years | — ($320) for 30 tablets | Weigh risks and benefits during pregnancy; no human data, although risk of fetal harm not expected based on human data with other allergen immunotherapy agents |
| House dust mite allergen extract (Odactra) | 18 years | — ($315) for 30 tablets | ||
| Five-grass pollen extract (Oralair) | 5 years | Only available at specialty pharmacies | ||
| Peanut allergen oral powder (Palforzia) | 4 years | — ($1,100) for one dose pack at maintenance dose | ||
| Short ragweed pollen extract (Ragwitek) | 5 years | — ($305) for 30 tablets | ||

| Type of therapy | Adverse effects |
|---|---|
| Intranasal corticosteroids | Bitter aftertaste, candidiasis, dry throat, epistaxis, headache, nasal irritation, nasal septum perforation, pharyngitis, rhinorrhea; minimal systemic absorption (50%) |
| Growth suppression in children has not been demonstrated with chronic use of nasal steroids22 | |
| Oral antihistamines | Dizziness, drowsiness, dry mouth, fatigue, headache |
| Anticholinergic properties of first-generation antihistamines cause antidyskinetic, antiemetic, and sedative effects | |
| Cetirizine (Zyrtec) and chlorpheniramine can cause sedation in children without a subjective feeling of drowsiness23 | |
| Diphenhydramine can cause paradoxical hyperactivity in children | |
| Diphenhydramine and loratadine do not cause sedation or affect school performance in children24 | |
| Intranasal antihistamines | Bitter aftertaste, headache, nasal irritation, sneezing, upper respiratory tract infection symptoms, xerostomia |
| Combination intranasal corticosteroid and antihistamine | Bitter aftertaste, candidiasis, dry throat, epistaxis, headache, nasal irritation, nasal septum perforation, pharyngitis, rhinorrhea, sneezing, upper respiratory tract infection symptoms, xerostomia; minimal systemic absorption (50%) |
| Growth suppression in children has not been demonstrated with chronic use of nasal steroids22 | |
| Oral decongestants | Anxiety, central nervous system stimulation, dizziness, elevated blood pressure, headache, palpitations, tremor, urinary retention |
| Intranasal anticholinergics | Dry mouth and throat, epistaxis, nasal irritation, taste changes, upper respiratory tract infection symptoms |
| Intranasal cromolyns | Bad taste, epistaxis, nasal burning, sneezing |
| Leukotriene receptor antagonists | Abdominal pain, headache, reflux, upper respiratory tract infection symptoms; FDA boxed warning for rare but serious neuropsychiatric disorders |
| Immunotherapy | Headache, itching, lip swelling, throat irritation; possible severe hypersensitivity reactions |
OTHER TREATMENTS
High-efficiency particulate air (HEPA) filters are ineffective at decreasing allergy symptoms.41,42
Bed, pillow, and quilt covers do not significantly decrease symptoms or the need for medication in adults with allergic rhinitis or asthma who are allergic to dust mites.43,44
The usefulness of inferior turbinate surgery to treat allergic rhinitis after failed medical treatment has not been determined.45
Homeopathic immunotherapy is ineffective for house dust mite allergy in adults with asthma.46
One RCT of 125 patients showed that butterbur was non-inferior to antihistamines in managing symptoms, but this study was deemed insufficient to make a guideline recommendation.2 Butterbur is considered safe by the National Institutes of Health only if pyrrolizidine alkaloids are removed.46
Prognosis
One study showed that 9.7% of patients with allergic rhinitis will develop asthma.47
It was believed that sensitization to potential allergens throughout life improved allergic rhinitis symptoms, but recent literature shows that allergic rhinitis tends to be misdiagnosed and is problematic for older and younger patients.48
This article updates previous articles on this topic by Sur and Plesa49 and Sur and Scandale.50
Data Sources: A PubMed search was conducted using the terms rhinitis, allergic, perennial and rhinitis, allergic, seasonal and rhinitis, and vasomotor. These terms were used to search the Agency for Healthcare Research and Quality, Essential Evidence Plus, and the Cochrane Database of Systematic Reviews. Search dates: December 10, 2020; April 28, 2022; and December 2022.
