
Am Fam Physician. 2023;107(5):539-540
Author disclosure: No relevant financial relationships.
Clinical Question
What is the most effective medical therapy for preventing postpartum hemorrhage after vaginal delivery?
Evidence-Based Answer
Oxytocin plus misoprostol is more effective than oxytocin alone in reducing postpartum hemorrhage after vaginal delivery. (Strength of Recommendation [SOR]: A, network meta-analysis of a randomized controlled trial [RCT] and subsequent large RCT.) However, this combination causes more nausea and vomiting than oxytocin alone. Tranexamic acid plus oxytocin does not significantly reduce the rate of postpartum hemorrhage compared with oxytocin alone. (SOR: B, large RCT.) Overall, oxytocin is the medication of choice for the prevention of postpartum hemorrhage because of the balance of high effectiveness and low incidence of adverse effects. (SOR: C, expert opinion.)
Evidence Summary
A systematic review and network meta-analysis involving 137 RCTs (many multinational) and 87,466 women examined uterotonic medications for the prevention of postpartum hemorrhage and ranked their effectiveness.1 Trials examined oxytocin, ergometrine (not available in the United States; similar to methylergonovine), misoprostol, and carbetocin (not available in the United States; similar to oxytocin) as single agents and the combinations of oxytocin plus misoprostol and oxytocin plus ergometrine. Patients delivered by vaginal birth or cesarean section. The medication was administered prophylactically by a systemic route: sublingually, subcutaneously, intramuscularly, rectally, orally, via intravenous bolus, or intravenous infusion; the timing of administration varied. These interventions were compared with other uterotonics, placebo, or no treatment. Two medication combinations—misoprostol plus oxytocin and ergometrine plus oxytocin—were more effective than standard oxytocin but had higher rates of nausea and vomiting (Table 1).1 A subanalysis of data from vaginal deliveries only (85 trials, N not provided) yielded similar reductions in hemorrhages of 500 mL or greater compared with oxytocin alone (relative risk [RR] of oxytocin plus misoprostol = 0.74; 95% CI, 0.56 to 0.99; and RR of oxytocin plus ergometrine = 0.69; 95% CI, 0.56 to 0.84). Most trials (71%) had a high risk or unclear risk of bias.
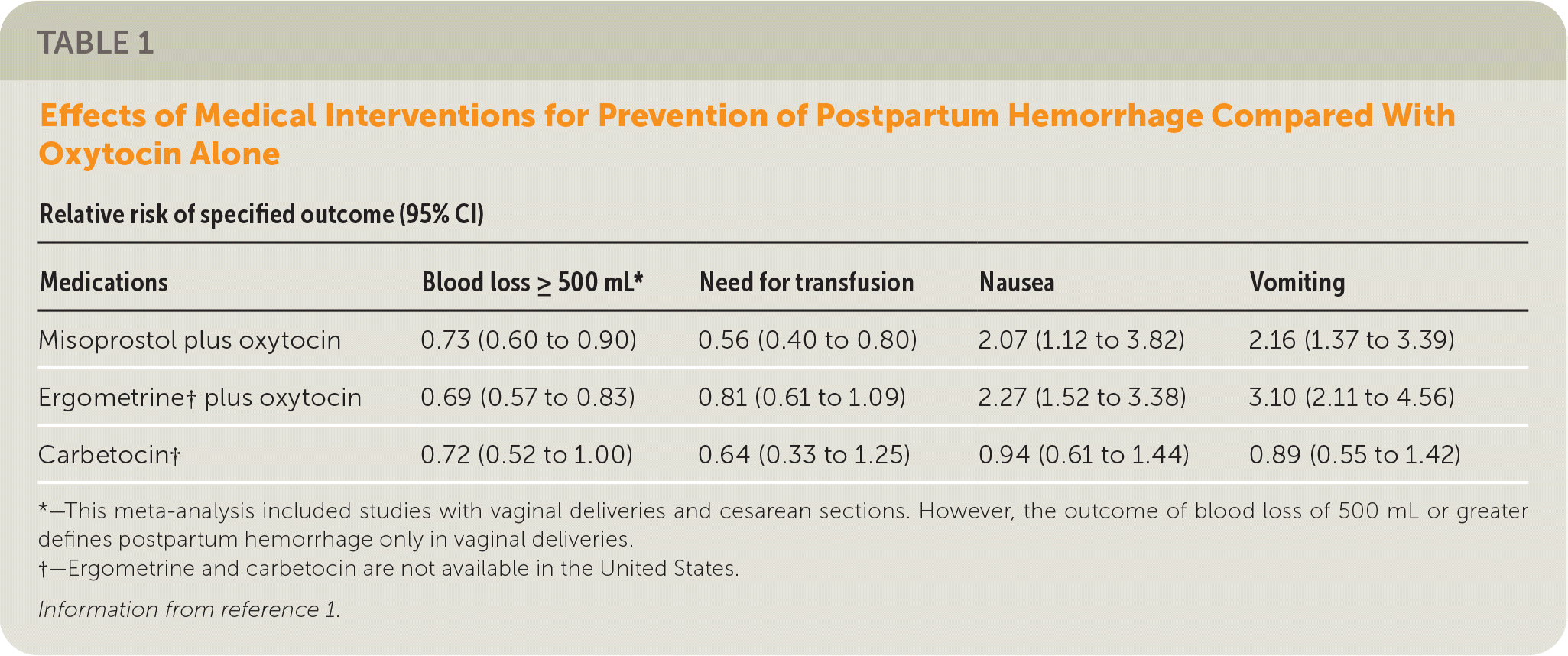
| Relative risk of specified outcome (95% CI) | ||||
|---|---|---|---|---|
| Medications | Blood loss ≥ 500 mL* | Need for transfusion | Nausea | Vomiting |
| Misoprostol plus oxytocin | 0.73 (0.60 to 0.90) | 0.56 (0.40 to 0.80) | 2.07 (1.12 to 3.82) | 2.16 (1.37 to 3.39) |
| Ergometrine† plus oxytocin | 0.69 (0.57 to 0.83) | 0.81 (0.61 to 1.09) | 2.27 (1.52 to 3.38) | 3.10 (2.11 to 4.56) |
| Carbetocin† | 0.72 (0.52 to 1.00) | 0.64 (0.33 to 1.25) | 0.94 (0.61 to 1.44) | 0.89 (0.55 to 1.42) |
A subsequent multicenter randomized trial in England compared the effectiveness of intramuscular oxytocin, carbetocin, and Syntometrine (a fixed combination of oxytocin and ergometrine) in the prevention of primary postpartum hemorrhage after vaginal delivery.2 Researchers enrolled 5,929 normotensive pregnant women older than 18 years who were beyond 24 weeks' gestation with a singleton pregnancy and were in early labor from six maternity units across England. Patients were randomized to 10 IU of oxytocin, 100 mcg of carbetocin, or 5 IU per 500 mcg of Syntometrine, all administered intramuscularly after placental expulsion. Patients receiving Syntometrine were significantly less likely to need additional uterotonics than patients given oxytocin (odds ratio [OR] = 0.75; 95% CI, 0.65 to0.91; P =.002) or carbetocin (OR = 0.78; 95% CI, 0.66 to 0.93; P = .004). The difference in hemorrhage between oxytocin and carbetocin was not statistically significant (19.5% vs. 19.1%; P = .78). Syntometrine, compared with carbetocin and oxytocin, resulted in more nausea (24%, 8%, and 8.9%, respectively), vomiting (17.6%, 4.8%, and 4.9%, respectively), hypertension (12.3%, 7.0%, and 7.1%, respectively), and trouble with infant bonding due to symptoms (8.4%, 2.9%, and 4.4%, respectively; P < .001 for all pairwise comparisons of Syntometrine with oxytocin and carbetocin).
A double-blind noninferiority RCT (n = 29,645) conducted across 23 sites in 10 countries compared the effectiveness of carbetocin vs. oxytocin in preventing postpartum hemorrhage.3 Researchers randomized patients to receive carbetocin, 100 mcg intramuscularly, or oxytocin, 10 IU intramuscularly, immediately after delivery of the infant. The two primary outcomes were the rate of blood loss of at least 500 mL or the need for additional uterotonic agents and a rate of blood loss of at least 1,000 mL. Patients who received carbetocin had a rate of postpartum hemorrhage of at least 500 mL or a need for uterotonics similar to those who received oxytocin (14.5% vs. 14.4%; RR = 1.01; 95% CI, 0.95 to 1.06). The rates of postpartum hemorrhage of at least 1,000 mL were also similar (1.51% for carbetocin vs. 1.45% for oxytocin; RR = 1.04; 95% CI, 0.87 to 1.25).
A recent RCT from France randomized 4,079 women delivering at 35 weeks' gestation or later to 1 g of intravenous tranexamic acid infusion over two minutes or placebo infusion in addition to a standard oxytocin injection upon delivery of the infant's anterior shoulder.4 The primary outcome was a postpartum hemorrhage of 500 mL or greater, measured in a collection bag. Among the 3,891 patients who delivered vaginally, the rate of postpartum hemorrhage was similar between the two groups (8.1% for tranexamic acid vs. 9.8% for placebo; RR = 0.83; 95% CI, 0.68 to 1.01; P = .07).
Recommendations From Others
In 2012, the World Health Organization advised using uterotonics in the third stage of labor to prevent postpartum hemorrhage and specifically recommended oxytocin, 10 IU administered intravenously or intramuscularly (strong recommendations, moderate-quality evidence).5 In 2017, the American College of Obstetricians and Gynecologists recommended that all obstetric care facilities have guidelines for the routine use of uterotonics, noting that oxytocin is “the most effective medication with the fewest adverse effects.”6
Copyright © Family Physicians Inquiries Network. Used with permission.
