Am Fam Physician. 2023;107(6):online
Author disclosure: No relevant financial relationships.

Details for This Review
Study Population: 8,805 patients with proven or suspected acute bacterial conjunctivitis
Efficacy End Points: Clinical effectiveness (i.e., clinical cure); resolution of at least two of the following three signs after a course of antibiotic therapy: bulbar conjunctival injection, palpebral conjunctival injection, and any purulent epiphora
Harm End Points: Antibiotic-associated ocular or systemic complications
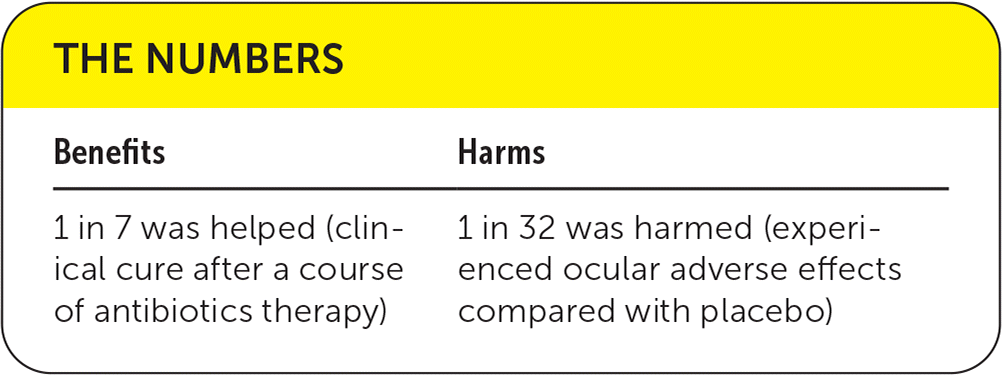
| Benefits | Harms |
|---|---|
| 1 in 7 was helped (clinical cure after a course of antibiotics therapy) | 1 in 32 was harmed (experienced ocular adverse effects compared with placebo) |
Physicians may treat acute infectious conjunctivitis with antibiotics more than needed because of the difficulty in clinical differentiation between bacterial and viral conjunctivitis, limited practical microbiological testing, and pressure from patients to return to work or school. Antibiotics may help hasten recovery and prevent serious complications (e.g., orbital cellulitis, keratitis, panophthalmitis); however, antibiotic therapy carries the risk of adverse effects and antibiotic resistance.3
A 2023 Cochrane review evaluated antibiotics for proven or suspected acute bacterial conjunctivitis (diagnosed clinically or microbiologically, with symptoms present for less than four weeks).4 The review included 21 randomized controlled trials (20 placebo-controlled trials), with 8,805 participants who were infants to 97 years of age, mostly in the United States (19 out of 21 trials). All interventions used topical antibiotics administered as ointment or drops, and 15 of the 21 studies examined fluoroquinolone drops. The dosing frequency ranged from twice per day to every two hours while awake (approximately eight times per day).
Moderate-certainty evidence showed that, compared with placebo, antibiotics were more likely to achieve a clinical cure (i.e., resolution of clinical symptoms or signs of infection) after a treatment course of varying duration (risk ratio [RR] = 1.26; 95% CI, 1.09 to 1.46; absolute risk difference [ARD] = 15%; number needed to treat = 7). A subgroup analysis showed no difference by antibiotic class (fluoroquinolone vs. nonfluoroquinolone) or treatment duration (three to five days vs. more than five days). Antibiotics also increased microbiological cure compared with placebo following randomization of those patients with a microbiologically proven diagnosis.
The Cochrane review provided very low-certainty evidence that nonf luoroquinolone antibiotics probably increased ocular adverse effects compared with placebo (RR = 4.05; 95% CI, 1.36 to 12; ARD = 0.031%; number needed to harm = 32; three trials, 556 participants), whereas topical fluoroquinolones had a lower risk than placebo (RR = 0.7; 95% CI, 0.54 to 0.9; four trials, 3,455 participants). There was no difference in systemic complications between antibiotic groups and placebo.
Caveats: The Cochrane review had several limitations. Pharmaceutical companies funded more than two-thirds of the trials. Out of 21 studies, 17 had a moderate to high overall risk of bias (mainly due to the randomization process) and were imprecise, affecting the certainty of evidence. Significant study heterogeneity resulted from different antibiotic preparations, patient populations, and methods of patient selection and determining primary outcomes. Although a microbiological cure was a reported disease-oriented outcome, it is not practical or cost-effective to perform this testing in all patients. Different treatment durations were also studied, and the use of antiseptic agents varied, limiting generalizability.
This Cochrane review found that 55% (408 out of 735) of participants in the placebo group had spontaneous clinical resolution by day 4 to 9, compared with 68.2% (504 out of 739) participants in the antibiotic groups. This is consistent with a previous systematic review and highlights the self-limiting nature of conjunctivitis.3 The American Academy of Ophthalmology does not recommend the routine use of antibiotics for acute conjunctivitis to avoid potential adverse effects because most cases are self-limiting.5 Cost-effectiveness should be considered because the management of bacterial conjunctivitis in the United States costs $857 million annually.6 However, family physicians should be cautious of hyperacute conjunctivitis, a similar condition with high morbidity and potential mortality from systemic involvement typically caused by Neisseria gonorrhoeae or Neisseria meningitidis.
Conclusion: Based on a high potential for bias, significant heterogeneity among trials, and limited generalizability, we have assigned a color recommendation of yellow (unclear benefits) to this review. Future research should standardize the treatment duration and focus on head-to-head trials between different antibiotics.