Am Fam Physician. 2023;107(6):661-664
Author disclosure: No relevant financial relationships.
Jaundice affects 4 out of 5 newborns, yet acute bilirubin encephalopathy and kernicterus are rare. Following a 2009 recommendation for universal newborn predischarge bilirubin screening, the incidence of hazardous bilirubin levels of 30 mg per dL (513 μmol per L) or greater decreased across three large health systems. The American Academy of Pediatrics (AAP) updated guidelines for the diagnosis and treatment of hyperbilirubinemia in infants born at more than 35 weeks of gestation.
Preventing Isoimmune Hemolytic Disease
Hemolysis is an important cause of hyperbilirubinemia in the newborn. Blood group typing, Rh(D) typing, and antibody screening should be performed early in pregnancy to determine the need for Rh(D) immune globulin and prevent isoimmune hemolytic disease of the newborn.
If screening was not performed during pregnancy, a maternal blood type and antibody screen should be obtained at admission. If the maternal antibody screen is positive or unknown at birth, a direct antiglobulin test and blood typing should be performed on the infant as soon as possible. Infants with negative direct antiglobulin test results are not at elevated risk and may be managed with usual care. Infants with a positive direct antiglobulin test are at increased risk of hemolysis and hyperbilirubinemia neurotoxicity unless their mother received Rh(D) immune globulin during pregnancy, because the immune globulin treatment can cause the positive direct antiglobulin test.
Hyperbilirubinemia Related to Feeding
Hyperbilirubinemia is common in infants who are exclusively breastfed but does not lead to hazardous hyperbilirubinemia. There are two main categories of jaundice associated with breastfeeding:
Breastfeeding jaundice is caused by suboptimal intake of calories and is commonly associated with excess weight loss. This type of jaundice presents earlier with a bilirubin peak on days 3 to 5.
Breast milk jaundice occurs in infants with adequate intake and weight gain. A component in breast milk can cause prolonged unconjugated hyperbilirubinemia that presents later and can persist for up to three months.
At 28 days of life, nearly 10% of infants have bilirubin concentrations of 10 mg per dL (171 μmol per L) or greater. To reduce the risk of hyperbilirubinemia, breastfeeding support should be provided within the first hour after birth and continued during the infant's first weeks of life. Parents should be encouraged to breastfeed infants at least eight times every 24 hours to ensure adequate intake. Oral supplementation with dextrose solution or water to prevent hyperbilirubinemia in breastfed infants is not recommended.
Identifying the Need for Treatment
The need for phototherapy, escalation of care, and exchange transfusion is determined using the infant's gestational age, the total serum bilirubin and hour of life obtained, and the presence of hyperbilirubinemia neurotoxicity risk factors. Those risk factors include birth before 38 weeks' gestation; clinical instability in the previous 24 hours; glucose-6-phosphate dehydrogenase (G6PD) deficiency or other hemolytic condition; infant albumin level less than 3 g per dL (30 g per L); positive direct antiglobulin test, if Rh(D) immune globulin was not given during pregnancy; and sepsis.
VISUAL ASSESSMENT
Visual assessment for jaundice offers an opportunity to identify early hyperbilirubinemia within 24 hours of birth, which is most often caused by hemolysis. The transcutaneous bilirubin or total serum bilirubin level should be measured immediately if infants are noted to be jaundiced within 24 hours of birth. After 24 hours, visual assessments should be used cautiously because visual estimates can differ from serum bilirubin by as much as 15 mg per dL (257 μmol per L).
G6PD DEFICIENCY
G6PD deficiency is rare but is one of the most important causes of hazardous hyperbilirubinemia and kernicterus. If a formula-fed infant has hyperbilirubinemia or if any infant has severe hyperbilirubinemia or late-onset jaundice, G6PD deficiency should be considered. G6PD activity should be measured in any infant with jaundice of unknown cause when bilirubin levels increase despite intensive phototherapy, increase suddenly, increase after an initial decline, or when escalated care is necessary. Measuring G6PD activity close to a hemolytic event or exchange transfusion can lead to a falsely normal result; therefore, repeat measurements may be necessary.
BILIRUBIN MEASUREMENT
Serum or transcutaneous bilirubin levels should be measured between 24 and 48 hours after birth or before discharge. Although transcutaneous bilirubin levels do not directly measure bilirubin, the measurement can identify infants who require a serum measurement. If the transcutaneous bilirubin level is 15 mg per dL or greater or within 3 mg per dL (51 μmol per L) of the phototherapy treatment threshold, a confirmatory serum bilirubin measurement should be obtained.
When bilirubin levels increase by 0.3 mg per dL (5.1 μmol per L) or greater per hour within 24 hours of birth or 0.2 mg per dL (3.4 μmol per L) or greater per hour after 24 hours, this suggests the presence of hemolysis. A rapid rate of increase can identify infants at higher risk of hyperbilirubinemia.
PROLONGED HYPERBILIRUBINEMIA
Prolonged hyperbilirubinemia suggests pathologic cholestasis. When breastfed infants are jaundiced at three to four weeks of life or formula-fed infants are jaundiced at two weeks of life, total and direct or conjugated bilirubin should be measured. Breastfed infants with direct or conjugated hyperbilirubinemia and formula-fed infants with any prolonged jaundice should be referred to gastroenterology for further evaluation. Although most breastfed infants with unconjugated hyper-bilirubinemia have breastmilk jaundice, newborn screening results should be reviewed for conditions such as galactosemia, hypothyroidism, and tyrosinemia.
Treatment
PHOTOTHERAPY
The phototherapy thresholds are higher than those in previous guidelines. Thresholds are determined through nomograms based on the absence (https://publications.aap.org/view-large/figure/13014081/PEDS_2022058859_f2.tif) and the presence (https://publications.aap.org/view-large/figure/13014082/PEDS_2022058859_f3.tif) of any neurotoxicity risk factors. Phototherapy should be initiated when the serum bilirubin exceeds the phototherapy threshold, which is based on the age of the infant in hours, the gestational age, and the presence of hyperbilirubinemia neurotoxicity risk factors. Phototherapy allows bilirubin to be more easily excreted and decreases the likelihood of an infant requiring escalated care, including exchange transfusion. Phototherapy has not been demonstrated to prevent the neurodevelopmental sequelae of hyper-bilirubinemia. Intensive phototherapy should be applied to as much of the infant's skin surface area as possible to optimize effectiveness.
HOME PHOTOTHERAPY
Home phototherapy can be used in infants with serum bilirubin levels above the phototherapy threshold after discharge if they meet the following criteria:
38 weeks or more of gestation
At least 48 hours of age
Clinically well appearing
Feeding adequately
No known hyperbilirubinemia neurotoxicity risk factors
Has not previously received phototherapy
Serum bilirubin concentration no more than 1 mg per dL (17 μmol per L) above phototherapy threshold
Serum bilirubin measured daily, if desired
Phototherapy devices available for home use without delay
An infant receiving home phototherapy should be admitted if the bilirubin level increases or becomes 1 mg per dL or greater above the photo-therapy threshold.
MONITORING DURING TREATMENT
For hospitalized infants, serum bilirubin should be measured within 12 hours after starting phototherapy. The timing and frequency of measurement can be guided by the age of the child, presence of hyperbilirubinemia neurotoxicity risk factors, bilirubin concentration, and rate of increase. Infants receiving home phototherapy should have serum bilirubin measured daily.
DISCONTINUING PHOTOTHERAPY
Discontinuing phototherapy is an option when the serum bilirubin level is at least 2 mg per dL (34 μmol per L) below the hour-specific threshold at which therapy was initiated. Although bilirubin values tend to increase after completion of phototherapy, the duration should be minimized to reduce the time infants are separated from their parents and limit adverse effects of the treatment. Extending phototherapy should be considered in infants at greater risk of further bilirubin increase, including phototherapy within 48 hours of birth, known hemolytic disease, and infants born before 38 weeks of gestational age.
ESCALATION OF CARE
Infants with a serum bilirubin level 2 mg per dL below the exchange transfusion threshold should be admitted to a neonatal intensive care unit with the capability of performing an exchange transfusion. Thresholds for exchange transfusion depend on whether the infant has risk factors (https://publications.aap.org/view-large/figure/13014116/PEDS_2022058859_f6.tif) or not (https://publications.aap.org/view-large/figure/13014115/PEDS_2022058859_f5.tif) for neurotoxicity. When escalating care, urgent measurement of total and direct serum bilirubin, serum albumin, and serum chemistries; complete blood count; blood typing; and crossmatch screening are required.
While care is escalated, serum bilirubin should be measured at least every two hours until the bilirubin level drops below the escalation-of-care threshold. Infants should receive intensive phototherapy and intravenous hydration if possible. Intravenous immune globulin can be considered in infants with isoimmune hemolytic disease.
Urgent exchange transfusion is needed for infants with serum bilirubin levels at or above the exchange transfusion threshold and any infant showing signs of acute bilirubin encephalopathy.
HOSPITAL DISCHARGE
Before hospital discharge, all families should receive education on neonatal jaundice. The last bilirubin measurement and the age at which it was measured, treatments provided, and important laboratory results should be given to the primary care physician or families to facilitate the transition of care.
Follow-up
AFTER PHOTOTHERAPY
Follow-up bilirubin testing after phototherapy is guided by the presence of risk factors for rebound hyperbilirubinemia. Serum bilirubin should be measured within six to 12 hours for infants who receive phototherapy within 48 hours of birth or have a positive direct antiglobulin test result or known or suspected hemolytic disease. All infants should have bilirubin measured the day after stopping phototherapy. Transcutaneous bilirubin testing should not be used until more than 24 hours after stopping phototherapy.
ROUTINE FOLLOW-UP
When discharging an infant who does not require treatment, the difference between the bilirubin concentration and the hour-specific phototherapy threshold should be used to determine the follow-up interval and the need for additional bilirubin measurements.
If the transcutaneous bilirubin level is within 3 mg per dL below the phototherapy treatment threshold, the serum bilirubin level should be measured. If that level is within 1.9 mg per dL (32.5 μmol per L) of the phototherapy threshold, a repeat serum bilirubin level should be measured in four to 24 hours, and discharge planning should be based on the age of the infant:
If younger than 24 hours, do not discharge and consider phototherapy.
If 24 hours or older, delay discharge and consider phototherapy, or discharge with close follow-up.
If 48 hours or older and other criteria are met, consider discharge with home phototherapy.
When the bilirubin concentration is 2 mg per dL or more below the hour-specific phototherapy threshold, follow-up depends on that difference:
If 2 to 3.4 mg per dL (34.2 to 58.2 μmol per L), measure serum or transcutaneous bilirubin in four to 24 hours.
If 3.5 to 5.4 mg per dL (59.9 to 92.4 μmol per L), measure serum or transcutaneous bilirubin in one to two days.
If 5.5 to 6.9 mg per dL (94.1 to 118.0 μmol per L), follow up in two days and measure serum or transcutaneous bilirubin if clinically indicated.
If 7 mg per dL (119.7 μmol per L) or greater, follow up in three days and measure serum or transcutaneous bilirubin if clinically indicated.
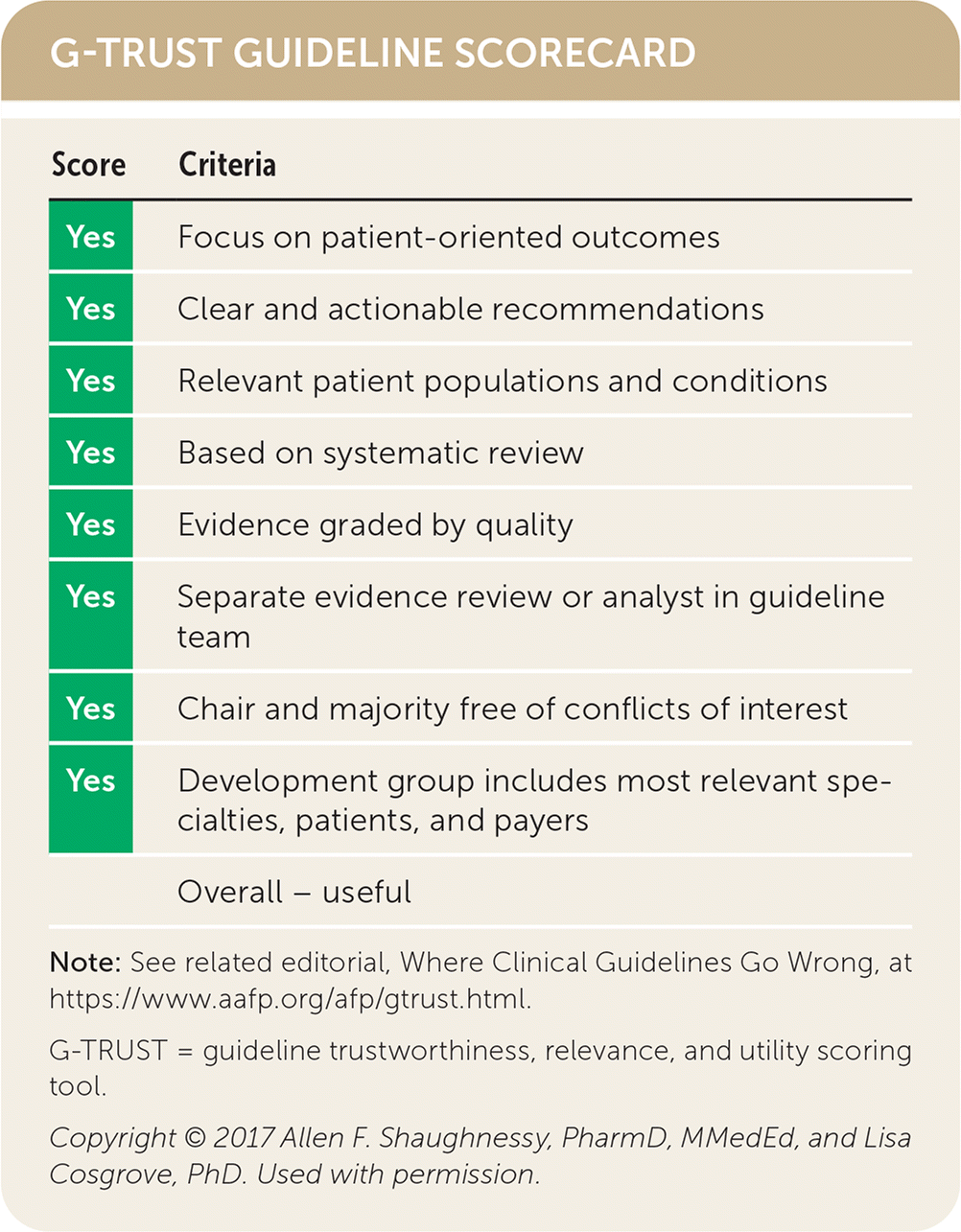
| Score | Criteria |
|---|---|
| Yes | Focus on patient-oriented outcomes |
| Yes | Clear and actionable recommendations |
| Yes | Relevant patient populations and conditions |
| Yes | Based on systematic review |
| Yes | Evidence graded by quality |
| Yes | Separate evidence review or analyst in guideline team |
| Yes | Chair and majority free of conflicts of interest |
| Yes | Development group includes most relevant specialties, patients, and payers |
| Overall – useful |
Editor's Note: This is the first complete update to guidelines for managing neonatal hyperbilirubinemia since 2004. Because kernicterus and bilirubin encephalopathy are exceedingly rare, the evidence behind this guideline is limited. The guideline committee included general pediatricians, a family physician, nurses, and breastfeeding experts, as well as experts in neonatal hyperbilirubinemia, which helped balance the priorities of minimizing separation of infants from their parents while protecting infants from the rare serious outcomes of hyperbilirubinemia. Although this guideline contains treatment nomograms, many clinicians use the calculator at https://www.bilitool.org instead, which has been updated with these new guidelines.—Steven R. Brown, MD, Contributing Editor
Dr. Brown was the AAFP representative to the 2022 Hyperbilirubinemia in Newborns: Updated Guidelines from the American Academy of Pediatrics clinical practice guideline committee.
Guideline source: American Academy of Pediatrics
Published source: Pediatrics. September 1, 2022; 150(3):e2022058859