
Am Fam Physician. 2023;108(2):119
Incorrect recommendation. In the article “Tuberculosis: Common Questions and Answers” (September 2022, p. 308), the recommended cutoff age for interferon-gamma release assay (IGRA) testing was listed incorrectly. In the first paragraph of the “What Are the Advantages and Disadvantages of TST vs. IGRA?” section, the second-to-last sentence (p. 309) should have read, “Disadvantages of IGRA testing include cost, and it is not recommended for use in children younger than two years.” An additional reference has been added to the online version of this article for this sentence and for a footnote in Table 2: Nolt D, Starke JR. Tuberculosis infection in children and adolescents: testing and treatment. Pediatrics. 2021;148(6):e2021054663. In Table 2 (p. 310), one of the cons listed for QuantiFERON-TB Gold+ should have read, “Not recommended for children younger than two years*” with the following accompanying footnote: “Guidelines from the Centers for Disease Control and Prevention and the American Thoracic Society state five years and older, but the American Academy of Pediatrics guidelines state two years and older.32” The online version of the article has been corrected.
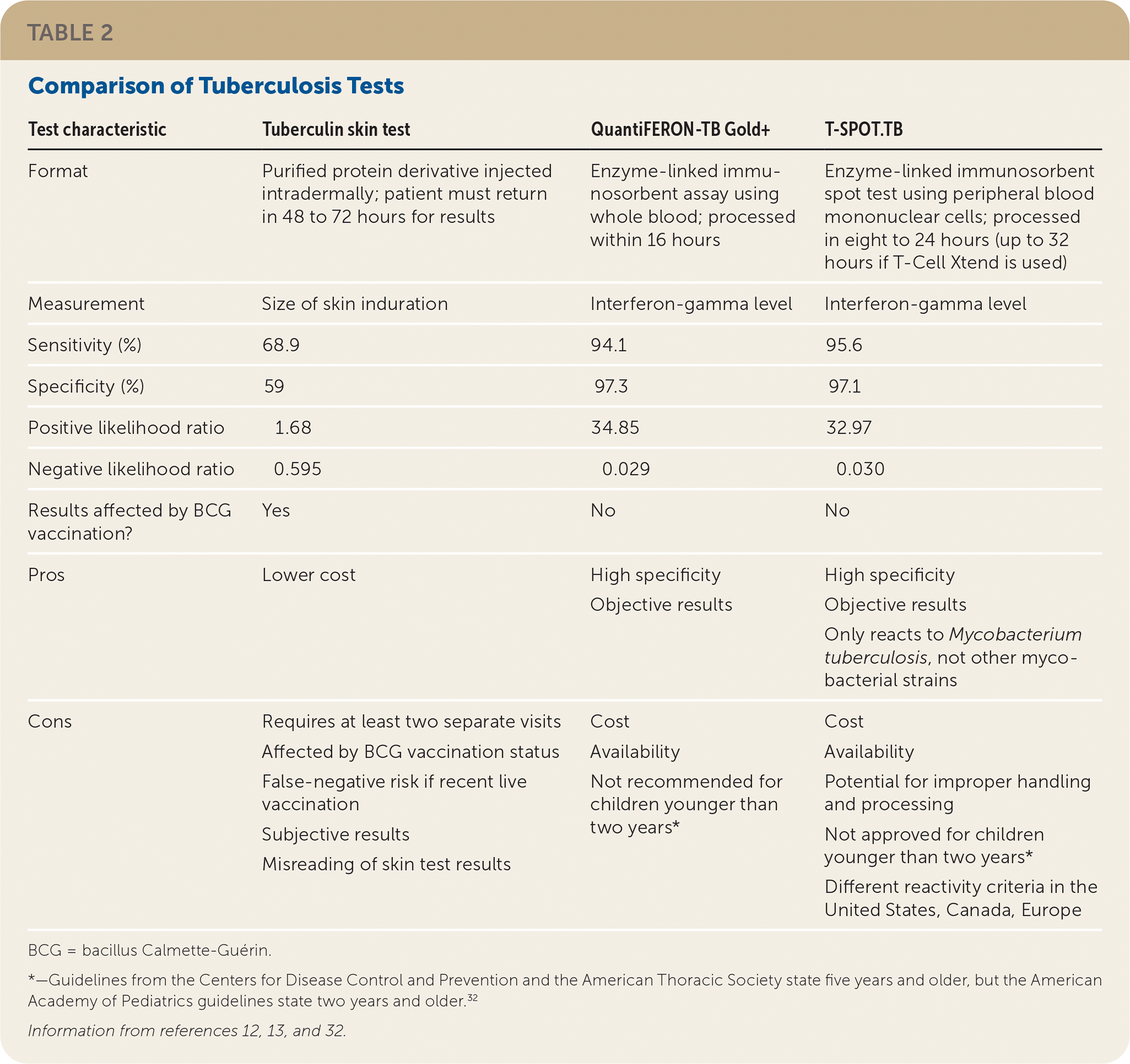
| Test characteristic | Tuberculin skin test | QuantiFERON-TB Gold+ | T-SPOT.TB |
|---|---|---|---|
| Format | Purified protein derivative injected intradermally; patient must return in 48 to 72 hours for results | Enzyme-linked immunosorbent assay using whole blood; processed within 16 hours | Enzyme-linked immunosorbent spot test using peripheral blood mononuclear cells; processed in eight to 24 hours (up to 32 hours if T-Cell Xtend is used) |
| Measurement | Size of skin induration | Interferon-gamma level | Interferon-gamma level |
| Sensitivity (%) | 68.9 | 94.1 | 95.6 |
| Specificity (%) | 59 | 97.3 | 97.1 |
| Positive likelihood ratio | 1.68 | 34.85 | 32.97 |
| Negative likelihood ratio | 0.595 | 0.029 | 0.030 |
| Results affected by BCG vaccination? | Yes | No | No |
| Pros | Lower cost | High specificity Objective results | High specificity Objective results Only reacts to Mycobacterium tuberculosis, not other mycobacterial strains |
| Cons | Requires at least two separate visits Affected by BCG vaccination status False-negative risk if recent live vaccination Subjective results Misreading of skin test results | Cost Availability Not recommended for children younger than two years* | Cost Availability Potential for improper handling and processing Not approved for children younger than two years* Different reactivity criteria in the United States, Canada, Europe |
