
Am Fam Physician. 2023;108(2):130-131
Author disclosure: No relevant financial relationships.
Details for This Review Study Population:
Adults 18 years or older with a diagnosis of symptomatic overactive bladder or detrusor overactivity
Efficacy End Points: Condition-specific quality of life; patient perception of cure or improvement; mean number of urgency episodes per 24 hours; mean number of micturitions per 24 hours
Harm End Points: Adverse events, including dry mouth and urinary retention; withdrawal from the trial due to adverse events
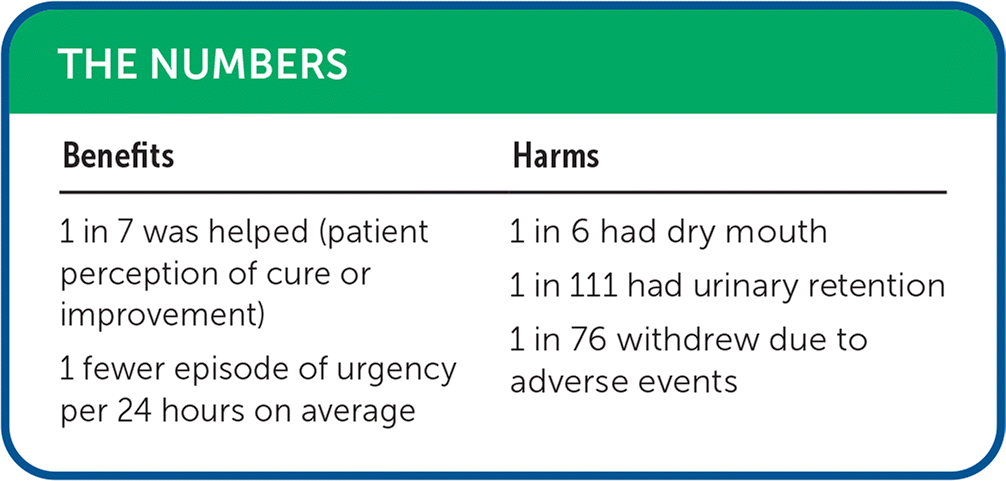
| Benefits | Harms |
|---|---|
| 1 in 7 was helped (patient perception of cure or improvement) 1 fewer episode of urgency per 24 hours on average | 1 in 6 had dry mouth 1 in 111 had urinary retention 1 in 76 withdrew due to adverse events |
Narrative: Overactive bladder describes a clinical syndrome of urinary urgency with or without incontinence that may be related to spontaneous contraction of the detrusor smooth muscle. Overactive bladder causes bothersome symptoms in 29.8 million U.S. adults 40 years and older, with women being affected nearly twice as often as men.1 Common treatment for overactive bladder includes oral anticholinergics, which are thought to work by blocking the muscarinic M3 receptors of the bladder's smooth muscle, causing relaxation; similar receptors are found throughout the body. Common adverse events from oral anticholinergics include dry mouth, constipation, dyspepsia, abdominal pain, urinary retention, urinary tract infection, dry eyes, blurred vision, headache, and dizziness. The Cochrane review discussed here examines the benefits and harms of anticholinergic drugs compared with placebo for the treatment of overactive bladder.2
This Cochrane review included 104 multinational or single-country studies (n = 47,106) comparing placebo with one of the following anticholinergic drugs at common clinical doses: darifenacin (Enablex), fesoterodine (Toviaz), imidafenacin (not available in the United States), oxybutynin, propantheline (not available in the United States), propiverine (not available in the United States), solifenacin (Vesicare), tolterodine, and trospium. Tolterodine was the most studied anticholinergic (38 trials). Evidence of other bladder outlet obstruction was a common criterion for exclusion.
For primary outcomes, anticholinergic drugs improved condition-specific quality of life compared with placebo (mean difference = −4.41; 95% CI, −5.28 to −3.54; n = 6,804; low-quality evidence). Patients treated with anticholinergics perceived a greater sense of cure or improvement (relative risk [RR] = 1.38; 95% CI, 1.15 to 1.66; absolute risk difference [ARD] = 16%; number needed to treat = 7; n = 8,457; moderate-quality evidence). They also experienced reduced episodes of urinary urgency per 24 hours (mean difference = −0.85; 95% CI, −1.03 to −0.67; n = 16,875; moderate-quality evidence).2
Participants in the anticholinergic group were more likely to withdraw from the trial due to adverse events (RR = 1.37; 95% CI, 1.21 to 1.56; ARD = 1.3%; number needed to harm [NNH] = 76; n = 36,943). Compared with placebo, anticholinergic drugs increased dry mouth (RR = 3.50; 95% CI, 3.26 to 3.75; ARD = 16%; NNH = 6; n = 38,368) and increased urinary retention (RR = 3.52; 95% CI, 2.04 to 6.08; ARD = 0.9%; NNH = 111; n = 7,862). The quality of evidence for all harm outcomes was low.2
Caveats: This Cochrane review included a large number of studies (104) examining nine different anticholinergic agents for the treatment of overactive bladder, with significant variation in the outcomes reported. The systematic review showed statistically significant change in patient-reported outcomes, but the effect sizes were modest. It is important to note that although anticholinergics were more effective than placebo for all key measurements, the placebo effect on outcomes was also significant, indicating the complexity of treating overactive bladder.
A potential limitation to the practical implication of these data is the lack of stratification of adverse events by age. As concerns for anticholinergic adverse events and burden increase with age, it is unclear whether these agents disproportionately cause withdrawal of treatment in older adults and, therefore, shift the balance from benefit to harm.
Conclusion: The 2019 American Urological Association guidelines for treatment of nonneurogenic overactive bladder in adults recommend behavior therapy as first-line treatment, with anticholinergics as second-line treatment or used in conjunction with behavior therapies. Extended-release formulations are recommended over immediate-release formulations to reduce dry mouth.3
We assigned a color recommendation of green (benefits greater than harms) to this treatment. Anticholinergics improve quality of life and patients' perception of cure and improvement, with a small increased risk of some adverse events. Overall withdrawal rates due to adverse events were low, and a focus on specific adverse events during counseling is warranted. Further studies can help stratify the risk of adverse events by age and with concurrent use of other anticholinergic agents. We recommend discussing with patients that using an oral anticholinergic for overactive bladder is likely to help—but also may have some significant adverse events.
