
Am Fam Physician. 2023;108(2):online
As published by the USPSTF.
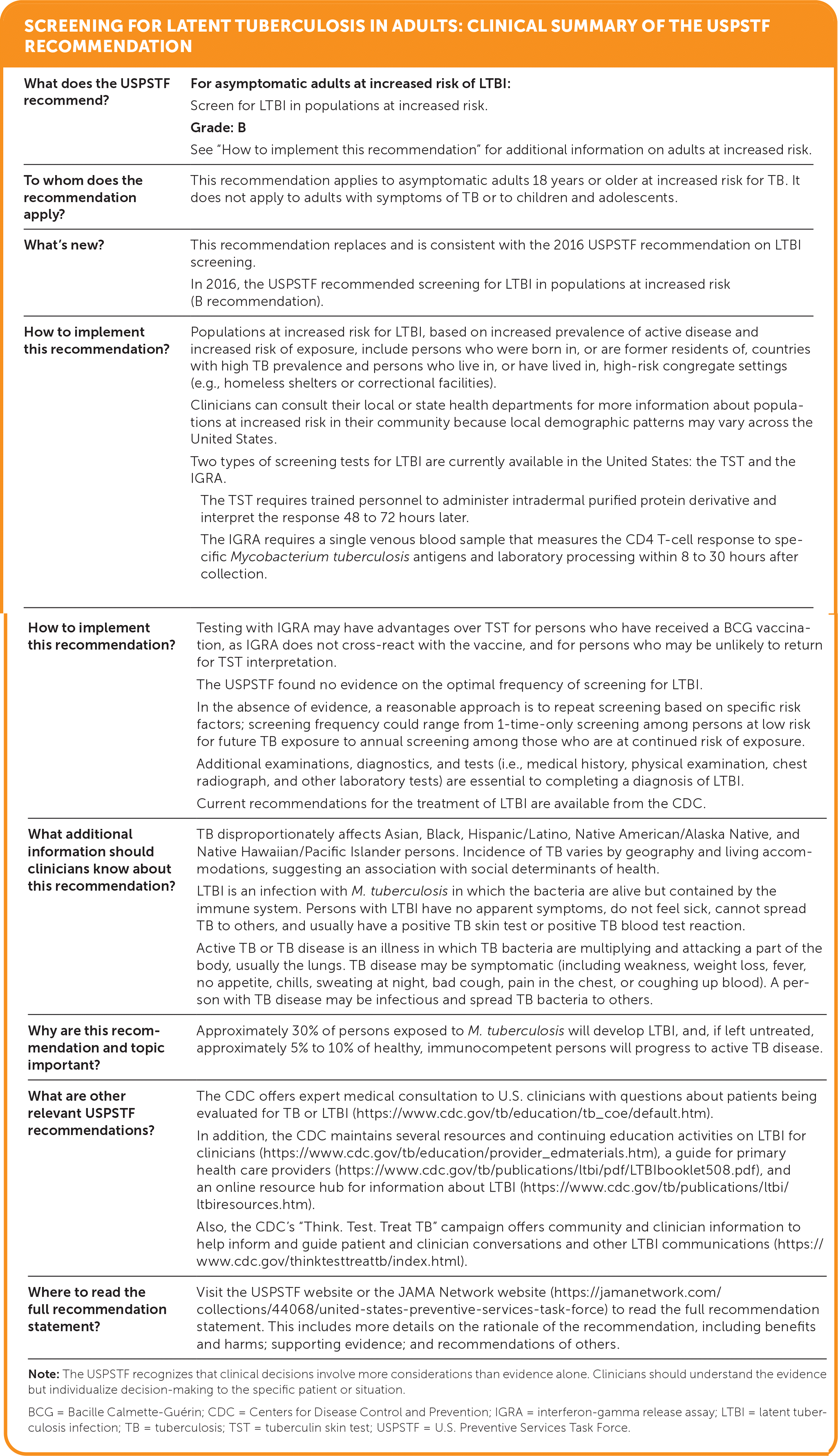
| What does the USPSTF recommend? | For asymptomatic adults at increased risk of LTBI: Screen for LTBI in populations at increased risk. Grade: B See “How to implement this recommendation” for additional information on adults at increased risk. |
| To whom does the recommendation apply? | This recommendation applies to asymptomatic adults 18 years or older at increased risk for TB. It does not apply to adults with symptoms of TB or to children and adolescents. |
| What's new? | This recommendation replaces and is consistent with the 2016 USPSTF recommendation on LTBI screening. In 2016, the USPSTF recommended screening for LTBI in populations at increased risk (B recommendation). |
| How to implement this recommendation? | Populations at increased risk for LTBI, based on increased prevalence of active disease and increased risk of exposure, include persons who were born in, or are former residents of, countries with high TB prevalence and persons who live in, or have lived in, high-risk congregate settings (e.g., homeless shelters or correctional facilities). Clinicians can consult their local or state health departments for more information about populations at increased risk in their community because local demographic patterns may vary across the United States. Two types of screening tests for LTBI are currently available in the United States: the TST and the IGRA. The TST requires trained personnel to administer intradermal purified protein derivative and interpret the response 48 to 72 hours later. The IGRA requires a single venous blood sample that measures the CD4 T-cell response to specific Mycobacterium tuberculosis antigens and laboratory processing within 8 to 30 hours after collection. |
| How to implement this recommendation? | Testing with IGRA may have advantages over TST for persons who have received a BCG vaccination, as IGRA does not cross-react with the vaccine, and for persons who may be unlikely to return for TST interpretation. The USPSTF found no evidence on the optimal frequency of screening for LTBI. In the absence of evidence, a reasonable approach is to repeat screening based on specific risk factors; screening frequency could range from 1-time-only screening among persons at low risk for future TB exposure to annual screening among those who are at continued risk of exposure. Additional examinations, diagnostics, and tests (i.e., medical history, physical examination, chest radiograph, and other laboratory tests) are essential to completing a diagnosis of LTBI. Current recommendations for the treatment of LTBI are available from the CDC. |
| What additional information should clinicians know about this recommendation? | TB disproportionately affects Asian, Black, Hispanic/Latino, Native American/Alaska Native, and Native Hawaiian/Pacific Islander persons. Incidence of TB varies by geography and living accommodations, suggesting an association with social determinants of health. LTBI is an infection with M. tuberculosis in which the bacteria are alive but contained by the immune system. Persons with LTBI have no apparent symptoms, do not feel sick, cannot spread TB to others, and usually have a positive TB skin test or positive TB blood test reaction. Active TB or TB disease is an illness in which TB bacteria are multiplying and attacking a part of the body, usually the lungs. TB disease may be symptomatic (including weakness, weight loss, fever, no appetite, chills, sweating at night, bad cough, pain in the chest, or coughing up blood). A person with TB disease may be infectious and spread TB bacteria to others. |
| Why are this recommendation and topic important? | Approximately 30% of persons exposed to M. tuberculosis will develop LTBI, and, if left untreated, approximately 5% to 10% of healthy, immunocompetent persons will progress to active TB disease. |
| What are other relevant USPSTF recommendations? | The CDC offers expert medical consultation to U.S. clinicians with questions about patients being evaluated for TB or LTBI (https://www.cdc.gov/tb/education/tb_coe/default.htm). In addition, the CDC maintains several resources and continuing education activities on LTBI for clinicians (https://www.cdc.gov/tb/education/provider_edmaterials.htm), a guide for primary health care providers (https://www.cdc.gov/tb/publications/ltbi/pdf/LTBIbooklet508.pdf), and an online resource hub for information about LTBI (https://www.cdc.gov/tb/publications/ltbi/ltbiresources.htm). Also, the CDC's “Think. Test. Treat TB” campaign offers community and clinician information to help inform and guide patient and clinician conversations and other LTBI communications (https://www.cdc.gov/thinktesttreattb/index.html). |
| Where to read the full recommendation statement? | Visit the USPSTF website or the JAMA Network website (https://jamanetwork.com/collections/44068/united-states-preventive-services-task-force) to read the full recommendation statement. This includes more details on the rationale of the recommendation, including benefits and harms; supporting evidence; and recommendations of others. |
The full recommendation statement is available at https://www.uspreventiveservicestaskforce.org/uspstf/recommendation/latent-tuberculosis-infection-screening.
The USPSTF recommendations are independent of the U.S. government. They do not represent the views of the Agency for Healthcare Research and Quality, the U.S. Department of Health and Human Services, or the U.S. Public Health Service.
