
Am Fam Physician. 2023;108(3):309-310
Author disclosure: No relevant financial relationships.
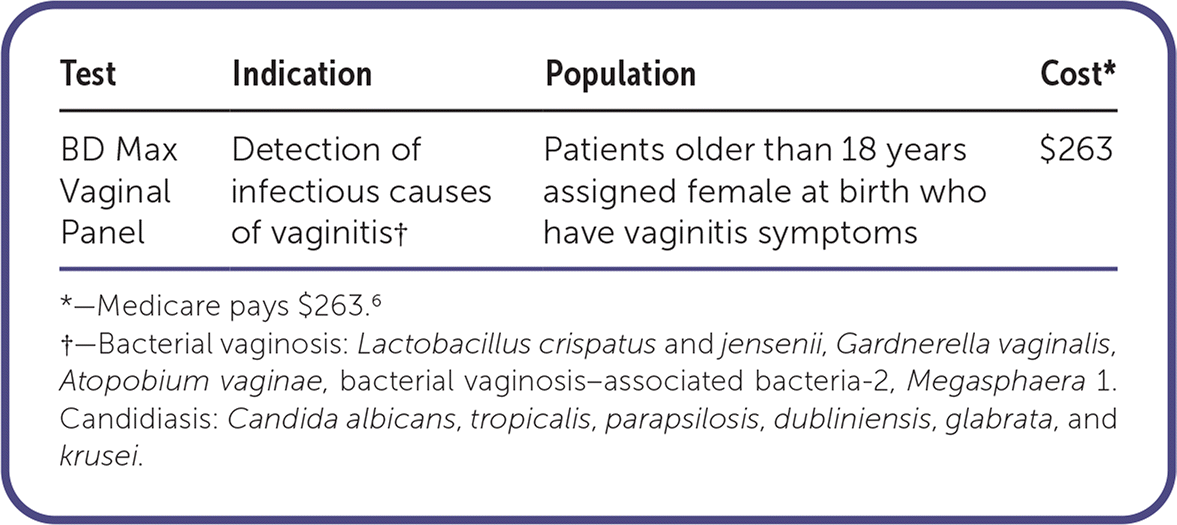
The BD Max Vaginal Panel is a nucleic acid amplification test (NAAT) that can detect the three main infectious causes of vaginitis (i.e., bacterial vaginosis organisms, Candida species, and Trichomonas vaginalis) in symptomatic adults assigned female sex at birth, including those who are pregnant. The BD Max Vaginal Panel should not be used for screening purposes.
This test was the first microbiome-based polymerase chain reaction assay approved by the U.S. Food and Drug Administration, although multiple similar NAATs are currently available.1 The American College of Obstetricians and Gynecologists recommends NAAT as the preferred diagnostic tool for trichomoniasis.2
Accuracy
A multicenter prospective clinical trial of 1,740 women presenting to 10 medical centers with vaginal symptoms compared the BD Max Vagi-nal Panel with clinician diagnosis and in-clinic testing (Amsel test, potassium hydroxide preparation, and wet mount).3 All testing methods were compared with reference methods of Nugent Gram stain for bacterial vaginosis, detection of the Candida gene ITS2, and T. vaginalis culture. The BD Max Vaginal Panel had a higher sensitivity than clinical diagnosis and in-clinic testing for all three organisms (Table 1).3
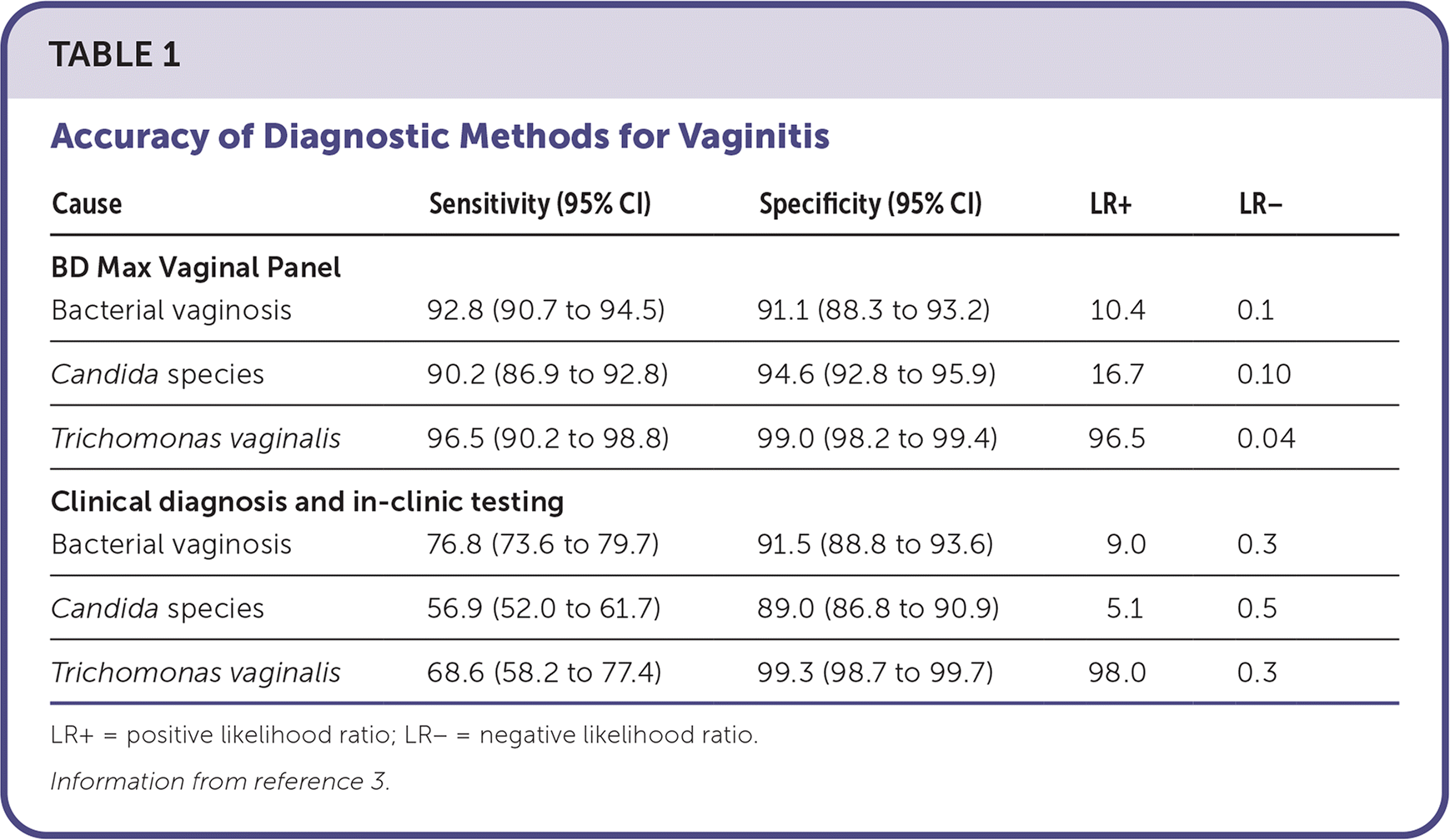
| Cause | Sensitivity (95% CI) | Specificity (95% CI) | LR+ | LR− |
|---|---|---|---|---|
| BD Max Vaginal Panel | ||||
| Bacterial vaginosis | 92.8 (90.7 to 94.5) | 91.1 (88.3 to 93.2) | 10.4 | 0.1 |
| Candida species | 90.2 (86.9 to 92.8) | 94.6 (92.8 to 95.9) | 16.7 | 0.10 |
| Trichomonas vaginalis | 96.5 (90.2 to 98.8) | 99.0 (98.2 to 99.4) | 96.5 | 0.04 |
| Clinical diagnosis and in-clinic testing | ||||
| Bacterial vaginosis | 76.8 (73.6 to 79.7) | 91.5 (88.8 to 93.6) | 9.0 | 0.3 |
| Candida species | 56.9 (52.0 to 61.7) | 89.0 (86.8 to 90.9) | 5.1 | 0.5 |
| Trichomonas vaginalis | 68.6 (58.2 to 77.4) | 99.3 (98.7 to 99.7) | 98.0 | 0.3 |
Foreign substances in the vagina, such as semen; blood; lubricant; contraceptive foams; and intravaginal antiviral, antifungal, and antibacterial medications can affect BD Max Vaginal Panel results. These substances can lead to unresolved, indeterminate, or false-negative results even in low concentrations. The BD Max Vaginal Panel will be unresolved or indeterminate approximately 3% of the time.1,4
Benefit
The BD Max Vaginal Panel is an easy-to-use vaginal swab that is collected in a clinician's office, placed in transport media, and evaluated in a laboratory. Self-collection is possible with appropriate guidance.1 It is an effective method for diagnosing common causes of vaginitis and may help guide more targeted management if speciation and sensitivities are necessary for effective treatment. The BD Max Vaginal Panel may also lead to fewer follow-up visits because it provides a conclusive diagnosis.5
Harms
This test has the potential to lead to over-treatment because detected organisms do not always require treatment. In-office microscopy and clinical analysis provide an immediate diagnosis, whereas the BD Max Vaginal Panel may lead to diagnostic and treatment delay depending on a variety of factors, including proximity to laboratory resources. It generally takes at least 24 hours to obtain results.
Cost
The Medicare reimbursement of BD Max Vaginal Panel is $263,6 whereas in-office microscopy costs about $5 to $10 in addition to the cost of obtaining and maintaining microscopy equipment.7 The BD Max Vaginal Panel is covered by most insurers. A cost-effectiveness study in women with private, employer-provided health insurance showed that patients who received an NAAT had more complete symptom resolution compared with traditional methods of diagnosis. This led to fewer follow-up visits and a 9% reduction in all-cause costs of treatment.5
Bottom Line
The BD Max Vaginal Panel is safe and accurately diagnoses most common bacterial, fungal, and protozoan causes of vaginitis in symptomatic women, including those who are pregnant. The cost of the vaginal panel is considerably higher than clinician diagnosis and traditional in-office microscopy. However, it provides diagnostic clarity, especially for vaginitis infections caused by Candida and Trichomonas species, and may reduce costs spent on ineffective treatment and multiple follow-up visits. The BD Max Vaginal Panel may be used selectively in symptomatic patients for supplemental diagnostic accuracy in conjunction with clinical judgment.
