
Am Fam Physician. 2023;108(3):online
Author disclosure: No relevant financial relationships.

Details for This Review
Study Population: 45,049 adult tobacco smokers who were willing to quit; varenicline (Chantix) studies were conducted in the United States and other countries, whereas cytisine studies were conducted in countries other than the United States; all studies were conducted in middle- and high-income countries and included inpatient and various outpatient settings
Efficacy End Points: Smoking abstinence at longest follow-up (six months or more)
Harm End Points: Serious adverse events, neuropsychiatric serious adverse events, and cardiac serious adverse events
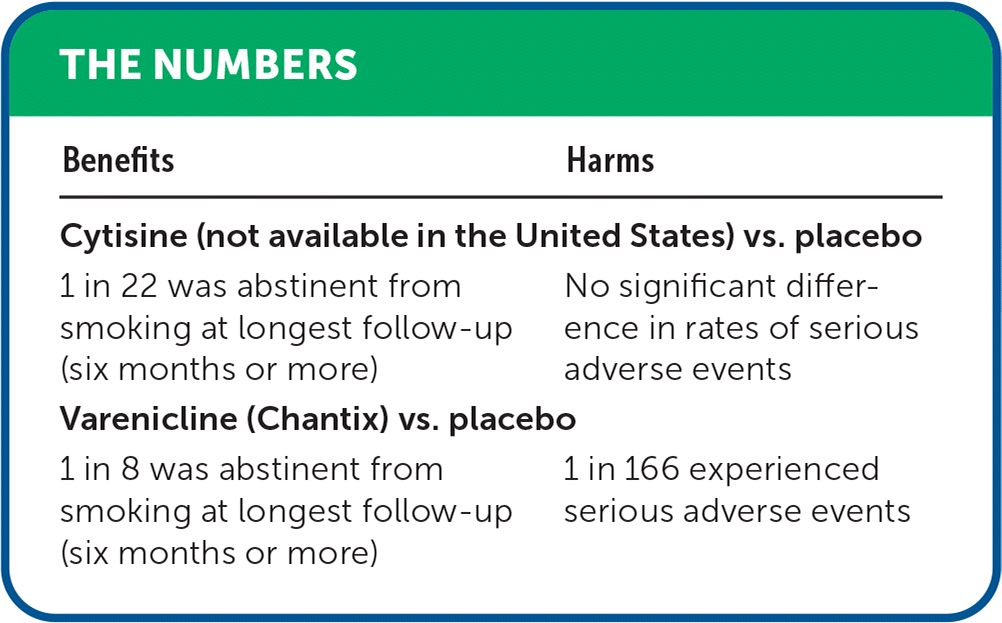
| Benefits | Harms |
|---|---|
| Cytisine (not available in the United States) vs. placebo | |
| 1 in 22 was abstinent from smoking at longest follow-up (six months or more) | No significant difference in rates of serious adverse events |
| Varenicline (Chantix) vs. placebo | |
| 1 in 8 was abstinent from smoking at longest follow-up (six months or more) | 1 in 166 experienced serious adverse events |
Narrative: Tobacco use is the leading cause of preventable death globally, with 1.3 billion users and more than 8 million deaths annually.1 In the United States, 18.7% of adults (46 million) reported using a tobacco product in 2021,2 and smoking and exposure to secondhand smoke cause an estimated 480,000 deaths annually.3 Although a variety of pharmacologic and behavioral interventions are available for smoking cessation, the rate of long-term abstinence remains low.4
A 2023 Cochrane review evaluated the effectiveness of varenicline compared with placebo, no medication, mono- and combination nicotine replacement therapy, bupropion, and cytisine, as well as the effectiveness and safety of cytisine as an alternative to varenicline for smoking cessation.5 The systematic review included 75 randomized controlled trials and cluster randomized controlled trials with 45,049 adult participants.
High-certainty evidence showed that varenicline helped more people quit smoking than placebo or no medication (risk ratio [RR] = 2.32; 95% CI, 2.15 to 2.51; absolute risk difference [ARD] = 13%; number needed to treat [NNT] = 8). Moderate-certainty evidence showed an increase in serious adverse events with varenicline (RR = 1.23; 95% CI, 1.01 to 1.48; ARD = 0.6%; number needed to harm [NNH] = 166) but no significant difference in rates of neuropsychiatric (e.g., suicidal ideation) or cardiac (e.g., arrhythmias, myocardial infarction) serious adverse events (low-certainty evidence).
High-certainty evidence showed that varenicline helped more people quit smoking than bupropion (RR = 1.36; 95% CI, 1.25 to 1.49; ARD = 6.4%; NNT = 16) with no significant difference in rates of serious adverse events, neuropsychiatric serious adverse events, or cardiac serious adverse events (all low-certainty evidence).
High-certainty evidence showed that varenicline helped more people quit smoking than nicotine replacement monotherapy (RR = 1.25; 95% CI, 1.14 to 1.37; ARD = 4.5%; NNT = 23), with fewer serious adverse events (RR = 0.70; 95% CI, 0.50 to 0.99; ARD = 0.3%; NNH = 333; low-certainty evidence).
Very low-certainty evidence in a single study showed that varenicline helped more people quit smoking than e-cigarettes (RR = 3.25; 95% CI, 1.21 to 8.71; ARD = 33%; NNT = 3).
Moderate-certainty evidence showed that cytisine helped more people quit smoking than placebo or no medication (RR = 1.30; 95% CI, 1.15 to 1.47; ARD = 4.7%; NNT = 22), with no significant difference in the rate of serious adverse events (low-certainty evidence). Low-certainty evidence also showed that cytisine helped more people quit smoking compared with mono- and combination nicotine replacement therapy (RR = 1.43; 95% CI, 1.13 to 1.80; ARD = 6.5%; NNT = 16).
There was no significant difference in quit rates between varenicline and combination nicotine replacement therapy or cytisine.
Caveats: The Cochrane review included 68 varenicline studies with approximately 37,000 participants. In addition, about 40% of the varenicline studies were conducted in the United States, making the results generalizable to the U.S. population. However, specific demographic factors limited generalizability to all smokers. Several studies included participants with potentially confounding medical conditions (e.g., cardiovascular disease, chronic obstructive pulmonary disease, HIV, substance or alcohol use disorders, depression). One study included those who previously tried and failed to quit smoking with varenicline. Two varenicline studies included light smokers (i.e., fewer than 10 cigarettes per day).
Compared with varenicline, there were fewer cytisine studies (eight) and participants (approximately 9,000). With cytisine not yet available in the United States, none of the included trials took place there. This limits generalizability even though cytisine was found to help people quit smoking compared with placebo, no medication, or nicotine replacement therapy.
Out of the 75 randomized controlled trials included in the review, the authors determined that 22 were at low risk of bias, 18 were at high risk, and 35 were at unclear risk. The risk of bias could potentially undermine the findings of the review. In addition, more than one-half of the studies received funding from pharmaceutical companies with interest in the medication being evaluated, received free study medication, or had authors who received funding from pharmaceutical companies for other work.
Conclusion: The existing evidence indicates that varenicline is more effective than placebo, no medication, bupropion, or nicotine replacement monotherapy for smoking cessation. People taking varenicline may experience more serious adverse events, although these are still considered rare.5 No definitive evidence supports a difference in quit rate between varenicline and cytisine or combination nicotine replacement therapy. Further studies conducted in the United States are needed to evaluate the effectiveness and safety of cytisine among the U.S. population.
Given the strength of evidence supporting varenicline effectiveness, the relative safety (low risk of serious adverse events), and promising initial evidence from international cytisine studies, we have assigned a color recommendation of green (benefits greater than harms) for the use of nicotine receptor partial agonists for tobacco cessation.
