
Am Fam Physician. 2023;108(4):424-426
Author disclosure: No relevant financial relationships.
Key Points for Practice
• Semaglutide is the most effective medication for weight loss and produces a total weight loss of 10% or more with a number needed to treat of 2 compared with lifestyle interventions alone.
• Phentermine/topiramate extended-release produces a total weight loss of 10% or more with a number needed to treat of 3 compared with lifestyle interventions alone.
• Phentermine monotherapy produces a total weight loss of 10% or more with a number needed to treat of 6 compared with lifestyle interventions alone.
From the AFP Editors
Nearly 42% of Americans, up from 30% in 2000, are obese. The limited effectiveness of lifestyle changes alone increases the need for effective, long-term treatment for those with a body mass index (BMI) greater than 30 kg per m2 or BMI of 27 kg per m2 or greater with associated complications (e.g., hypertension, diabetes mellitus, and hyperlipidemia). The American Gastroenterological Association performed a systematic review to publish guidelines for managing obesity with medications.
Most Effective Medications
The American Gastroenterological Association established 3% weight loss as the minimally important difference for determining effectiveness of medication over lifestyle interventions alone. For a person who weighs 90.7 kg (200 lb), this would be an excess weight loss of 2.7 kg (6 lb). Four medications meet that criterion.
GLUCAGON-LIKE PEPTIDE-1 AGONISTS
These subcutaneous injectable medications are effective for weight loss and aid in treating type 2 diabetes. Glucagon-like peptide-1 agonists are contraindicated for patients with a personal or family history of medullary thyroid cancer or multiple endocrine neoplasia syndrome type 2 and who are receiving treatment with dipeptidyl-peptidase-4 inhibitors. These medications have been associated with an increase in gallbladder disease, pancreatitis, and delayed gastric emptying that may contribute to nausea, vomiting, and postponement of absorption of other medications.
Semaglutide. Semaglutide (Ozempic), 2.4 mg injected subcutaneously weekly, with lifestyle interventions results in the greatest weight loss. Semaglutide produces an average of nearly 11% total body weight loss, and it has a number needed to treat (NNT) of 2 (95% CI, 2 to 4) for producing weight loss of 10% or more and an NNT of 3 (95% CI, 2 to 5) for producing weight loss of 15% or more. In patients with type 2 diabetes, semaglutide reduces A1C by at least 1.0%. Semaglutide leads to treatment discontinuation for adverse effects, with a number needed to harm (NNH) of 29 (95% CI, 17 to 58).
Liraglutide. Liraglutide (Saxenda) is a glucagon-like peptide-1 agonist with similar effects as semaglutide, but it has less apparent benefit. Treatment with liraglutide results in a 4.8% total body weight loss and has an NNT of 6 (95% CI, 5 to 9) for producing weight loss of 10% or more and an NNT of 11 (95% CI, 8 to 18) for producing a weight loss of 15% or more. In patients with type 2 diabetes, liraglutide reduces A1C by approximately 0.5%. Liraglutide leads to treatment discontinuation for adverse effects, with an NNH of 20 (95% CI, 14 to 30). Liraglutide requires a daily injection, whereas semaglutide requires a weekly injection.
COMBINATION ORAL MEDICATIONS
Phentermine/topiramate extended-release (ER; Qsymia) and naltrexone/bupropion ER (Contrave) are effective oral medications to consider if glucagon-like peptide-1 agonists are contraindicated, poorly tolerated, inaccessible, or not preferred.
Phentermine/topiramate ER. Phentermine/topiramate ER (maximum oral dosage of 15 mg/92 mg, once daily) produces an average 8.5% total body weight reduction and has an NNT of 3 (95% CI, 3 to 4) for producing weight loss of 10% or more and NNT of 4 (95% CI, 3 to 7) for producing weight loss of 15% or more. It can be useful for those with comorbid migraine disorder.
Phentermine/topiramate ER leads to treatment discontinuation for adverse effects, with an NNH of 10 (95% CI, 7 to 16). Despite the concern for increased blood pressure with phentermine monotherapy, improved blood pressure control was observed after phentermine/topiramate ER treatments. The medication is contraindicated in patients with uncontrolled hypertension, history of cardiovascular disease, or untreated hyperthyroidism. Topiramate is a teratogen, which requires that women have effective contraception if they use this medication. Taking phentermine/topiramate ER earlier in the day may limit insomnia.
Naltrexone/bupropion ER. Naltrexone/bupropion ER (maximum oral dosage of 16 mg/180 mg, twice daily) produces an anticipated average 3% total body weight reduction and has an NNT of 7 (95% CI, 4 to 18) for producing weight loss of 10% or more and an NNT of 12 (95% CI, 6 to 30) for producing weight loss of 15% or more. Naltrexone/bupropion ER may be useful in treating comorbid depression and tobacco use. Naltrexone (Revia) as monotherapy or in combination with any medication may interfere with acute pain management and perioperative treatment that requires opioids. Naltrexone/bupropion ER is contraindicated in patients with seizure disorders; individuals with hypertension should be monitored. Naltrexone/bupropion ER leads to treatment discontinuation for adverse effects, with an NNH of 7 (95% CI, 4 to 15).
Amphetamine Derivatives Are Somewhat Effective
Although approved for short-term use, sympathomimetic amines are often used for longer periods.
PHENTERMINE MONOTHERAPY
Phentermine monotherapy (oral dosage of 15 to 37.5 mg, once daily) is the most commonly used anti-obesity medication. Phentermine produces an average 3.6% total body weight reduction and has an NNT of 6 (95% CI, 3 to 11) for producing weight loss of 10% or more. Phentermine monotherapy leads to treatment discontinuation for adverse effects, with an NNH of 13 (95% CI, 8 to 27). Phentermine is contraindicated in patients with uncontrolled hypertension, history of cardiovascular disease, or untreated hyperthyroidism.
DIETHYLPROPION
Diethylpropion (maximum oral dosage of 75 mg controlled-release, once daily midmorning, or oral dosage of 25 mg immediate-release, three times daily with meals) produces an average 5.4% total body weight reduction and has an NNT of 4 (95% CI, 2 to 12) for producing weight loss of 10% or more. This medication is contraindicated in patients with uncontrolled hypertension, history of cardiovascular disease, or untreated hyperthyroidism. Common adverse effects include insomnia, irritability, and anxiety; adverse effects do not appear to be different from placebo in limited study.
Least Effective Medications
ORLISTAT
Orlistat inhibits gastrointestinal tract lipase and is available over the counter as 60-mg capsules or by prescription as 120-mg capsules. It is taken orally three times daily with meals. Orlistat produces an average 2.8% total body weight reduction and has an NNT of 7 (95% CI, 6 to 10) for producing weight loss of 10% or more. Discontinuation for adverse effects is not different from placebo. Adverse effects include flatulence, oily spotting, and fecal urgency and incontinence. Orlistat is contraindicated in patients with malabsorption diseases, such as pancreatic insufficiency. When using orlistat long term, consider supplementing the diet with fat-soluble vitamins. Orlistat may impair absorption of long-acting oral medications, such as levothyroxine or warfarin.
SUPERABSORBENT HYDROGEL
Gelesis100 is a superabsorbent hydrogel that is dosed as three capsules (2.25 g per dose) taken with water before lunch and dinner. The capsules absorb water and grow into a space-occupying matrix within the gut that increases satiety and decreases food consumption. Unlike intragastric balloons that require procedural removal, the gel is degraded in the colon and excreted. A single study suggests that the gel results in an average of 2% weight loss, less than the minimum criterion.
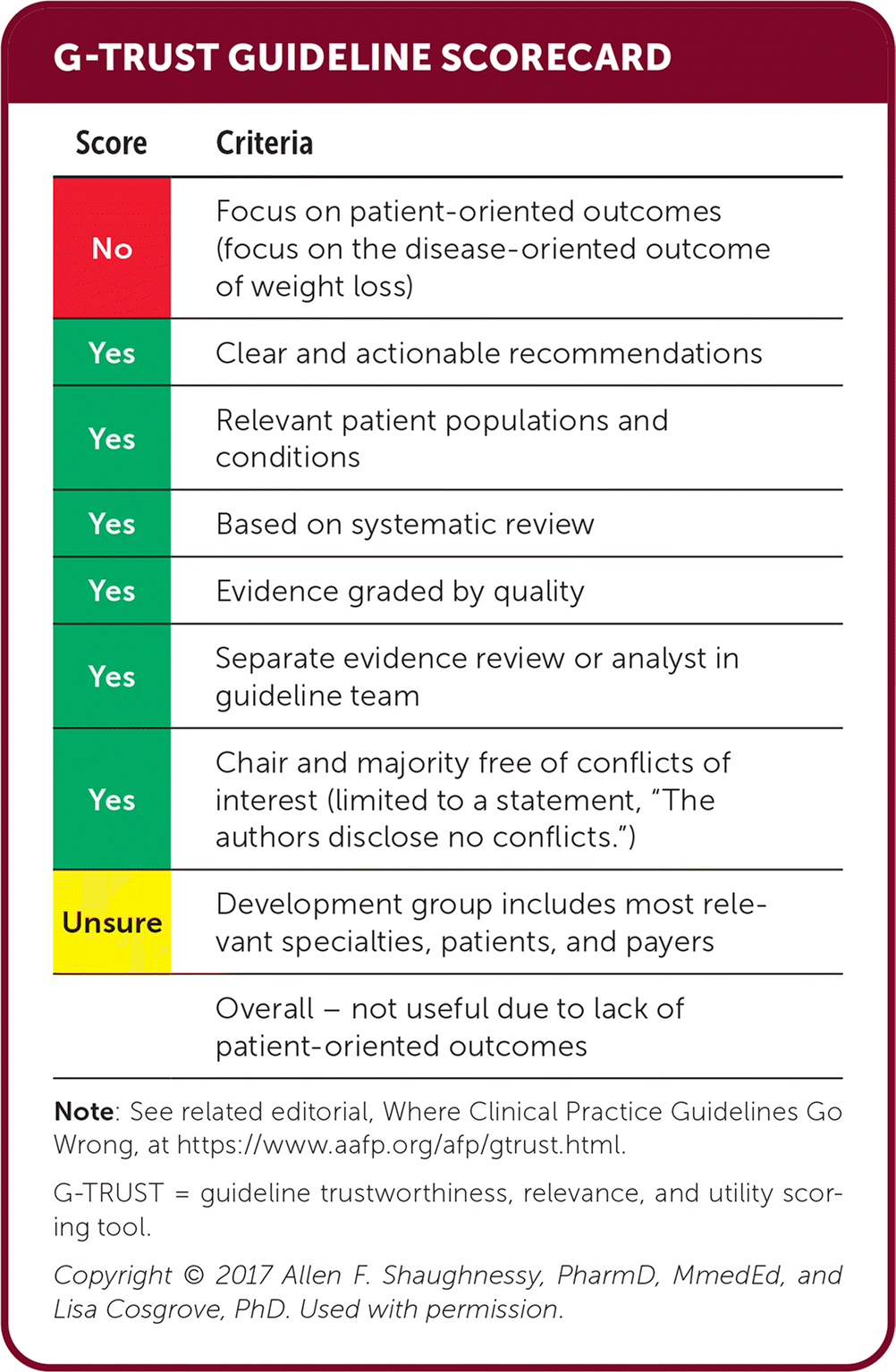
| Score | Criteria |
|---|---|
| No | Focus on patient-oriented outcomes (focus on the disease-oriented outcome of weight loss) |
| Yes | Clear and actionable recommendations |
| Yes | Relevant patient populations and conditions |
| Yes | Based on systematic review |
| Yes | Evidence graded by quality |
| Yes | Separate evidence review or analyst in guideline team |
| Yes | Chair and majority free of conflicts of interest (limited to a statement, “The authors disclose no conflicts.”) |
| Unsure | Development group includes most relevant specialties, patients, and payers |
| Overall – not useful due to lack of patient-oriented outcomes |
Editor's Note: This guideline lacks patient-oriented evidence of benefit; however, the summary of data on weight loss medications is advantageous. Although many of these treatments are unaffordable to many of our patients, treatment with phentermine or off-label use of phentermine and topiramate as separate prescriptions may increase the availability.—Michael J. Arnold, MD, Assistant Medical Editor
The numbers needed to treat and to harm and related CIs reported in this Practice Guideline were calculated by the author based on raw data provided in the original guideline.
The views expressed are those of the author and do not necessarily reflect the official policy or position of the Uniformed Services University of the Health Sciences, U.S. Navy, U.S. Department of Defense, or U.S. government.
Guideline source: American Gastroenterological Association
Published source: Grunvald E, Shah R, Hernaez R, et al. AGA clinical practice guideline on pharmacological interventions for adults with obesity. Gastroenterology. 2022;163(5):1198–1225.
