
Am Fam Physician. 2023;108(4):420-422
Author disclosure: No relevant financial relationships.
Published online September 18, 2023.
Key Points for Practice
• One dose of nirsevimab is recommended for infants up to eight months of age born during or entering their first RSV season, and for children between eight and 19 months of age who remain vulnerable to severe RSV disease through their second RSV season.
• A single-dose RSV vaccine can be offered to adults 60 years and older after shared decision-making.
• The influenza vaccine is recommended for all people older than six months, with no additional safety measures for egg allergies because there is minimal risk of a reaction.
• All children should receive either the 20- or 15-valent PCV between two and 23 months of age.
From the AFP Editors
In the United States, respiratory syncytial virus (RSV) infection causes seasonal epidemics of respiratory illness, leading to severe symptoms, lower respiratory tract disease, hospitalization, and death in infants and older adults. RSV infection is one of the most common causes of childhood illness, and it is the most common cause of hospitalization in infants, with up to 80,000 hospitalizations and 300 deaths occurring annually in children younger than five years.
Older adults, especially those 75 years and older, who are frail, live in long-term care facilities, or have medical conditions such as immunosuppression, diabetes mellitus, and chronic lung, kidney, and cardiovascular disease are at increased risk for RSV-associated hospitalization. In older U.S. adults, up to 160,000 hospitalizations and 10,000 deaths due to RSV occur annually.
Seasonal RSV epidemics, when 3% or more of RSV polymerase chain reaction test results are positive, usually begin in October, peak in December, and end in April. Since the second year of the COVID-19 pandemic, RSV seasons have started and peaked earlier. Timing of the upcoming season is uncertain.
RSV Prevention in Children
The Advisory Committee on Immunization Practices (ACIP) recommends a single dose of nirsevimab (Beyfortus), a long-acting monoclonal antibody, for passive immunization to prevent RSV-associated lower respiratory tract disease in infants up to eight months of age born during or entering their first RSV season, and in children eight to 19 months of age who remain vulnerable to severe RSV disease through their second RSV season (Table 1). Nirsevimab is associated with an 80% relative risk reduction in hospitalization and a 90% relative risk reduction for intensive care unit admission for RSV disease in the first RSV season without increasing serious adverse effects compared with placebo.
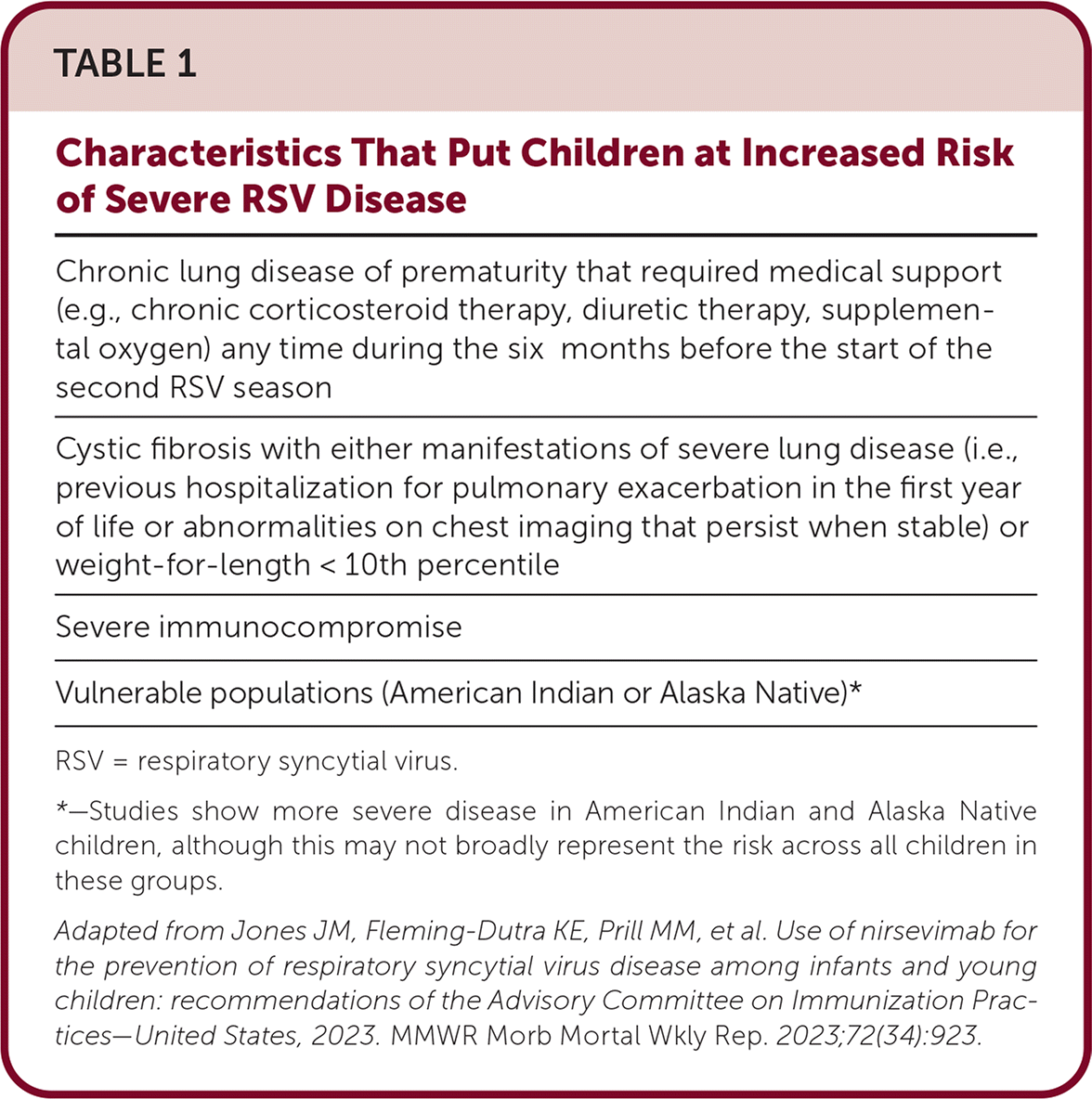
| Chronic lung disease of prematurity that required medical support (e.g., chronic corticosteroid therapy, diuretic therapy, supplemental oxygen) any time during the six months before the start of the second RSV season |
| Cystic fibrosis with either manifestations of severe lung disease (i.e., previous hospitalization for pulmonary exacerbation in the first year of life or abnormalities on chest imaging that persist when stable) or weight-for-length < 10th percentile |
| Severe immunocompromise |
| Vulnerable populations (American Indian or Alaska Native)* |
Use of the previous monoclonal antibody, palivizumab, to prevent severe RSV disease among infants and young children has been limited by high cost and the requirement for monthly dosing.
The recommended dose of nirsevimab is 50 mg for infants weighing less than 5 kg (11 lb), 100 mg for infants weighing 5 kg or more during the first RSV season, and 200 mg administered as two 100-mg injections given at the same time at different injection sites for children eight to 19 months of age at increased risk for severe RSV disease and entering their second RSV season. Coadministration of nirsevimab with routine age-appropriate vaccines is recommended. Nirsevimab is not expected to interfere with the immune response to other routine childhood immunizations.
RSV Prevention in Adults
The ACIP recommends offering a single-dose RSV vaccine for adults 60 years and older following shared decision-making. Unlike routine and risk-based vaccine recommendations, recommendations based on shared decision-making do not target all persons in a particular age group or an identifiable risk group. Characteristics of adults at highest risk for severe RSV disease are listed in Table 2.
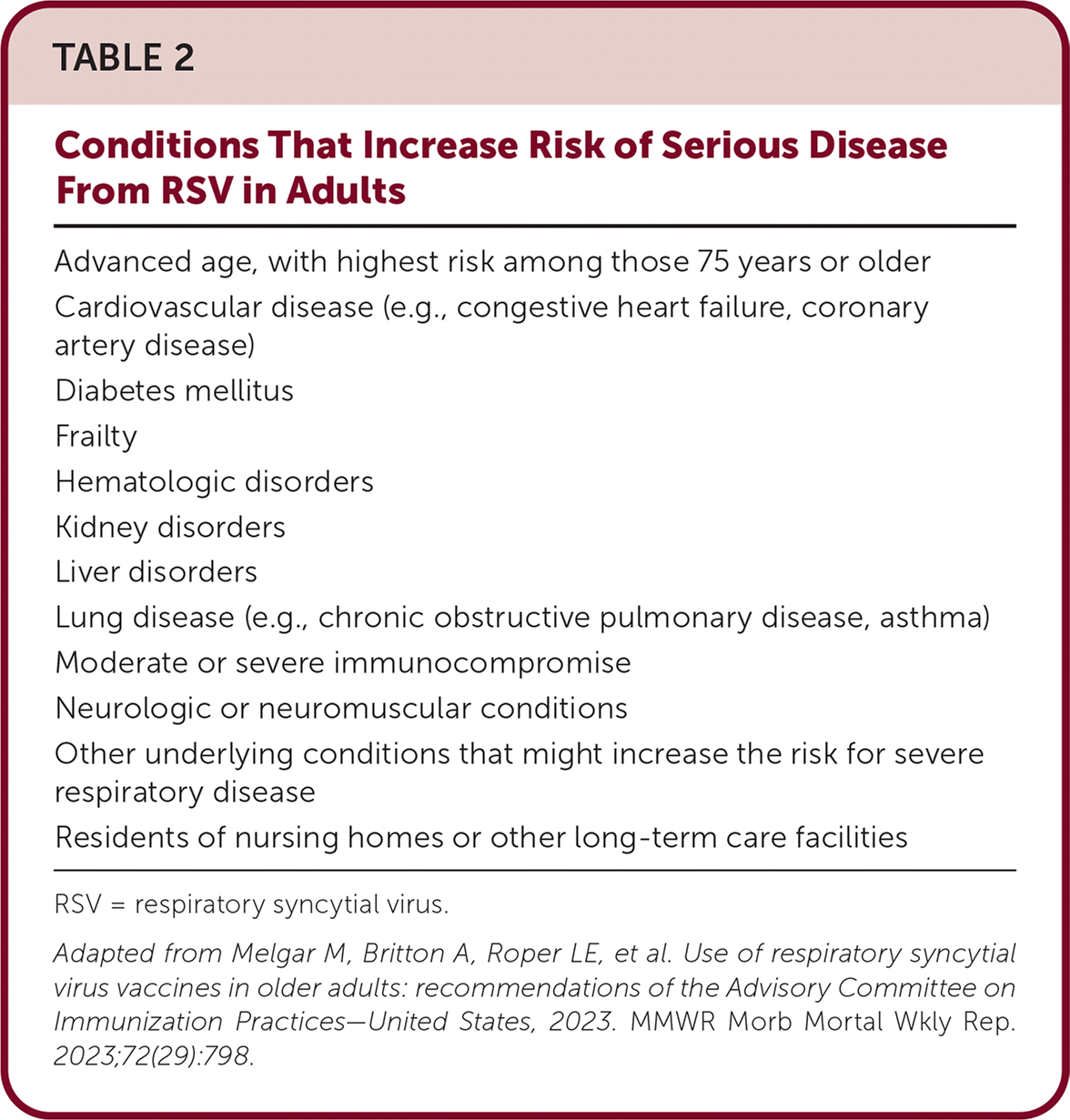
| Advanced age, with highest risk among those 75 years or older |
| Cardiovascular disease (e.g., congestive heart failure, coronary artery disease) |
| Diabetes mellitus |
| Frailty |
| Hematologic disorders |
| Kidney disorders |
| Liver disorders |
| Lung disease (e.g., chronic obstructive pulmonary disease, asthma) |
| Moderate or severe immunocompromise |
| Neurologic or neuromuscular conditions |
| Other underlying conditions that might increase the risk for severe respiratory disease |
| Residents of nursing homes or other long-term care facilities |
Either of the RSV vaccines for adults can be used: RSVPreF3 (Arexvy), an adjuvanted recombinant stabilized prefusion F protein vaccine, or RSVpreF (Abrysvo), a recombinant stabilized prefusion F vaccine. RSV vaccines can be given with other immunizations, including influenza, although data on coadministration are limited. Both vaccines offer a relative risk reduction of 75% or more in lower respiratory tract infections for two years after immunization. Studies show a low rate of adverse effects, including atrial fibrillation and inflammatory neurologic events, including Guillain-Barré syndrome, that did not reach statistical significance. Postmarketing safety surveillance is ongoing.
Influenza Vaccination
For the 2023-2024 season, ACIP continues to recommend the influenza vaccine for everyone six months and older in the United States. September and October remain the optimal times for most people to get vaccinated. Vaccination in July and August should be considered for pregnant people in the third trimester and children with routine health care visits during these months to avoid missing the opportunity to vaccinate them.
The 2023–2024 vaccine includes an updated inf luenza A(H1N1)pdm09 component: A/Victoria/4897/2022 (H1N1)pdm09-like virus for egg-based vaccines and A/Wisconsin/67/2022 (H1N1)pdm09-like virus for cell-based or recombinant vaccines. The ACIP recommends that people with egg allergy can receive either egg- or non–egg-based influenza vaccines without additional safety measures because there is minimal risk of a reaction. More information on influenza vaccine recommendations can be accessed at https://www.cdc.gov/flu/spotlights/2022-2023/flu-vaccination-recommendations-adopted.htm.
Pneumococcal Vaccination
In June 2023, the ACIP recommended the 20-valent pneumococcal conjugate vaccine (PCV) for children and adults. It also expanded the definition of high-risk children to include those with moderate persistent and severe persistent asthma. Either the 20- or 15-valent PCV should be used for all children two to 23 months of age, following recommended pneumococcal dosing schedules. Either the 20- or 15-valent PCV should be used for catch-up vaccination in children 24 to 71 months of age with an incomplete PCV status or specific high-risk conditions previously recommended for 13-valent PCV immunization.
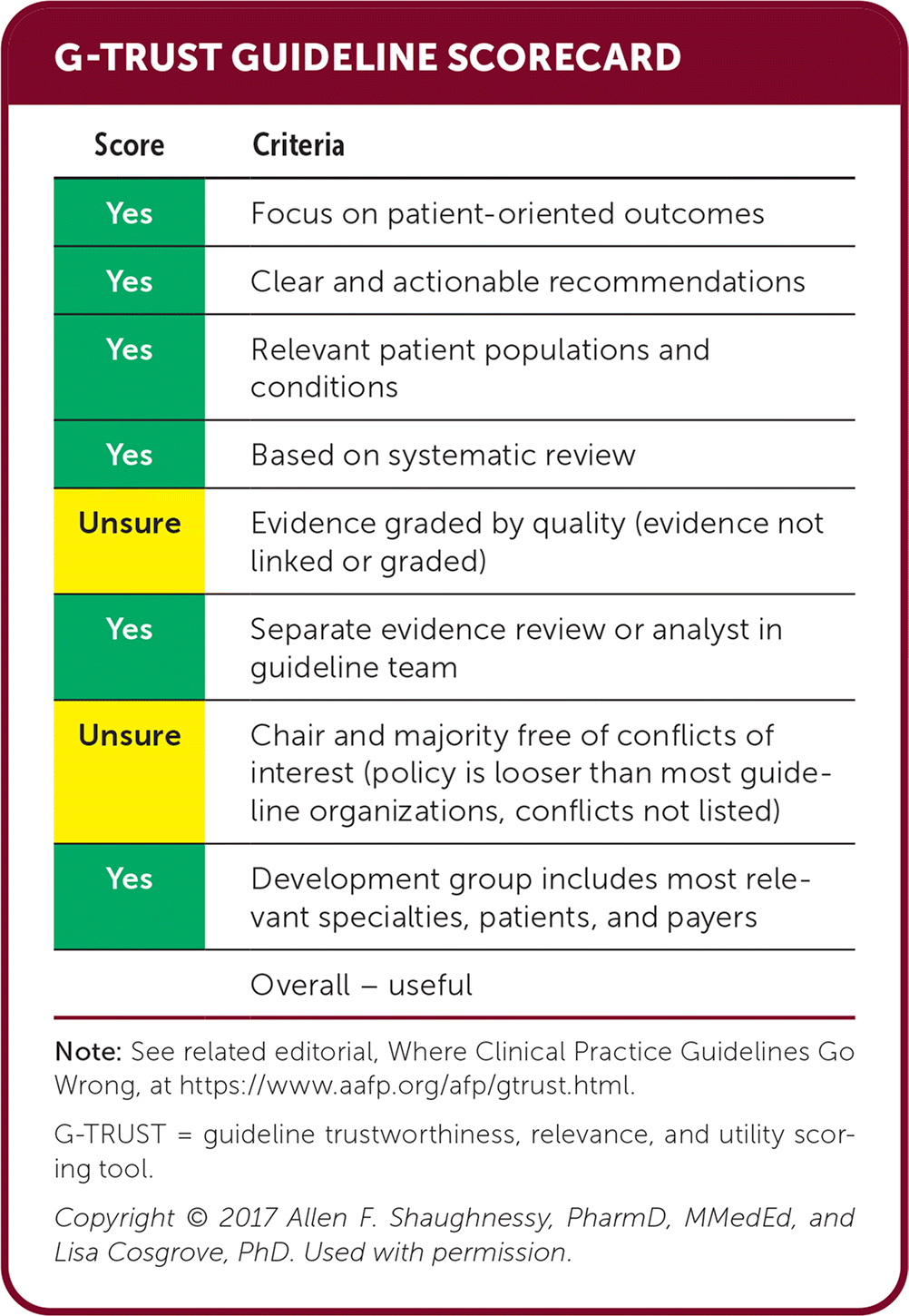
| Score | Criteria |
|---|---|
| Yes | Focus on patient-oriented outcomes |
| Yes | Clear and actionable recommendations |
| Yes | Relevant patient populations and conditions |
| Yes | Based on systematic review |
| Unsure | Evidence graded by quality (evidence not linked or graded) |
| Yes | Separate evidence review or analyst in guideline team |
| Unsure | Chair and majority free of conflicts of interest (policy is looser than most guideline organizations, conflicts not listed) |
| Yes | Development group includes most relevant specialties, patients, and payers |
| Overall – useful |
Editor's Note: Dr. Rockwell serves as liaison to ACIP for the AAFP.
The new ACIP recommendations have several important changes, including shifting pneumococcal vaccination to the 20- and 15-valent protein conjugated vaccines. The option to vaccinate older adults for RSV is another interesting recommendation. One of the most dramatic and possibly controversial recommendations is passive immunization with nirsevimab for all children in their first RSV season (October to February). A Centers for Disease Control and Prevention model estimates a number needed to immunize of 130 to prevent one hospitalization during the first RSV season.1—Michael J. Arnold, MD, Assistant Medical Editor
Reference
1. Ortega-Sanchez IR. Economics of preventing respiratory syncytial virus lower respiratory tract infections (RSV-LRTI) among US infants with nirsevimab. Accessed September 1, 2023. https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2023-02/slides-02-23/rsv-pediatric-03-sanchez-508.pdf
Guideline source: Advisory Committee on Immunization Practices
Published source: Jones JM, Fleming-Dutra KE, Prill MM, et al. Use of nirsevimab for the prevention of respiratory syncytial virus disease among infants and young children: recommendations of the Advisory Committee on Immunization Practices—United States, 2023. MMWR Morb Mortal Wkly Rep. 2023;72(34):920–925, and Melgar M, Britton A, Roper LE, et al. Use of respiratory syncytial virus vaccines in older adults: recommendations of the Advisory Committee on Immunization Practices— United States, 2023. MMWR Morb Mortal Wkly Rep. 2023;72(29):793–801.
