
Am Fam Physician. 2023;108(5):523-526
Author disclosure: No relevant financial relationships.
Key Points for Practice
• For acute or chronic pain not related to cancer, sickle cell disease, or end-of-life care, consider nonpharmacologic and nonopioid pharmacologic treatments before prescribing opioids.
• Other diagnoses and alternative treatments should be considered before initiating opioid therapy or continuing for more than 30 days.
• When discontinuing opioid therapy, a slow taper can minimize withdrawal symptoms. Slow tapers are often less than 10% of the daily dosage per month, especially after use for one year or more.
From the AFP Editors
Approximately 1 in 14 adults reported having pain that limited their life and work on most days during the past three months. Chronic pain impairs physical functioning, mental health, and quality of life. Nearly 1 in 10 people who commit suicide had evidence of chronic pain at the time of death.
Black, Hispanic, and Asian people are less likely to be assessed or treated for pain. White, American Indian, and Alaska Native people have a higher risk of prescription opioid–related overdose deaths; however, safeguards and monitoring are more often applied to Black patients.
The 2016 Centers for Disease Control and Prevention (CDC) opioid prescribing guidelines resulted in laws, regulations, and policies that were unintended and often exceeded the clinical recommendations by including cancer and palliative care, rapid opioid tapers, and rigid thresholds that limited the treatment of opioid use disorder and led to patient dismissal and abandonment by physicians. The CDC has released new recommendations for prescribing opioids.
Given the continued overuse of opioids to treat pain, the guidelines continue to suggest limiting their use in patients with acute or chronic pain. The guidelines do not apply to children or patients with sickle cell disease or cancer-related pain or who are receiving palliative or end-of-life care.
Alternatives to Opioids for Pain
Table 1 outlines nonpharmacologic therapies for acute and chronic pain.
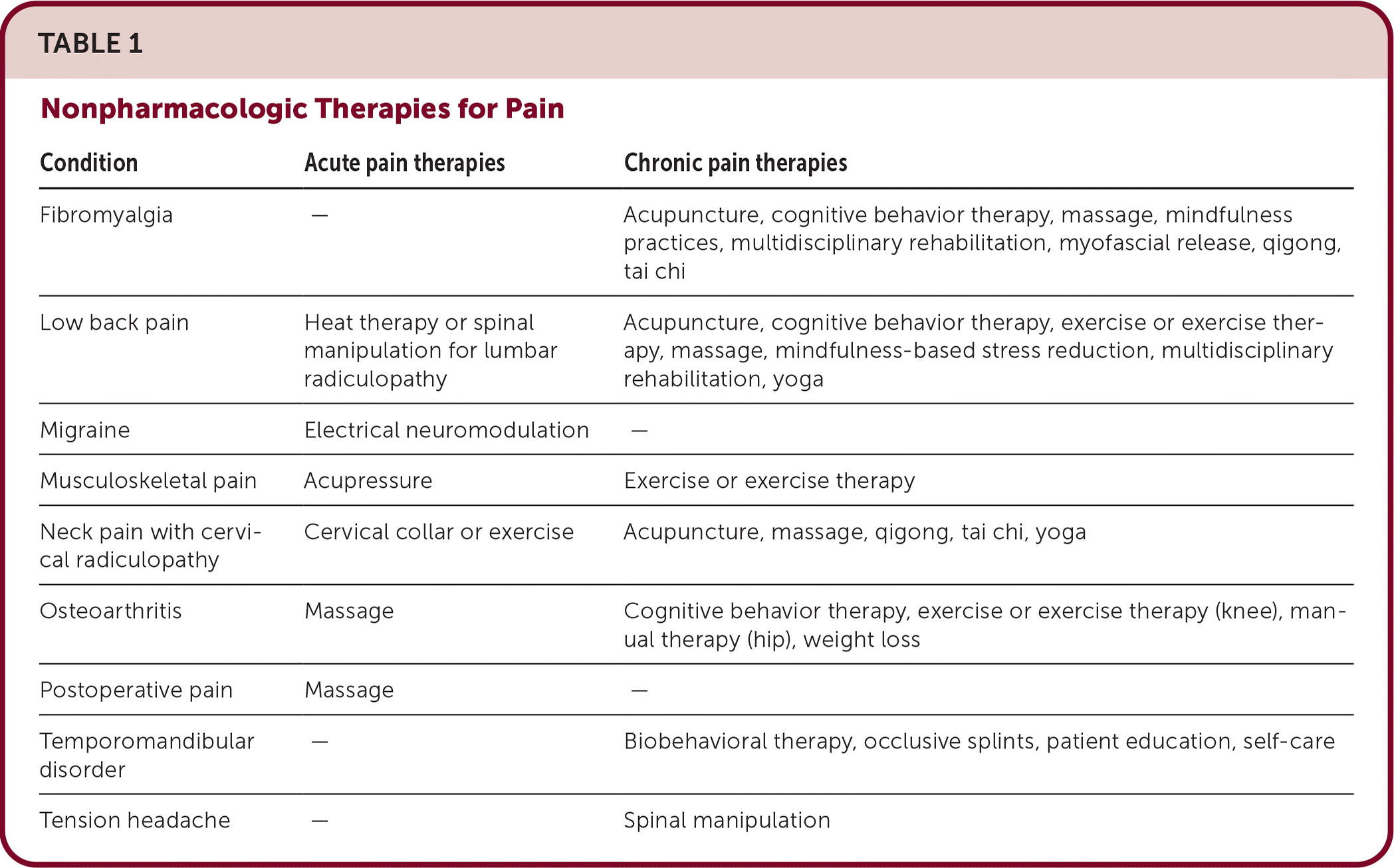
| Condition | Acute pain therapies | Chronic pain therapies |
|---|---|---|
| Fibromyalgia | — | Acupuncture, cognitive behavior therapy, massage, mindfulness practices, multidisciplinary rehabilitation, myofascial release, qigong, tai chi |
| Low back pain | Heat therapy or spinal manipulation for lumbar radiculopathy | Acupuncture, cognitive behavior therapy, exercise or exercise therapy, massage, mindfulness-based stress reduction, multidisciplinary rehabilitation, yoga |
| Migraine | Electrical neuromodulation | — |
| Musculoskeletal pain | Acupressure | Exercise or exercise therapy |
| Neck pain with cervical radiculopathy | Cervical collar or exercise | Acupuncture, massage, qigong, tai chi, yoga |
| Osteoarthritis | Massage | Cognitive behavior therapy, exercise or exercise therapy (knee), manual therapy (hip), weight loss |
| Postoperative pain | Massage | — |
| Temporomandibular disorder | — | Biobehavioral therapy, occlusive splints, patient education, self-care |
| Tension headache | — | Spinal manipulation |
ACUTE PAIN
Multiple nonpharmacologic therapies and modalities can be used to relieve acute pain. These approaches can be used alone or in addition to nonopioid medications. Some treatments are not covered by medical insurance programs, and the CDC encourages health insurers to provide or increase payment for these therapies.
Nonopioid medications are at least as effective as opioids, and nonsteroidal anti-inflammatory drugs (NSAIDs) are recommended as primary therapy for many conditions. Topical NSAIDs are effective for most musculoskeletal pain conditions, especially those close to the skin. Systemic NSAIDs are effective for musculoskeletal, dental, kidney stone, and migraine pain. Migraines can be treated with systemic NSAIDs, with or without migraine-specific acute treatments such as triptans.
CHRONIC PAIN
As with acute pain, chronic pain can be improved by many nonpharmacologic and nonopioid pharmacologic treatments without exposing patients to the risk of opioids.
Most chronic musculoskeletal pain, such as low back pain and osteoarthritis, responds to NSAIDs. Topical NSAIDs are effective for joints near the skin. Systemic NSAIDs or duloxetine (Cymbalta) can be effective for many types of musculoskeletal and neuropathic pain, although both medications can be limited by contraindications and adverse effects. Tricyclic antidepressants, other serotonin-norepinephrine reuptake inhibitors, selected anticonvulsants, capsaicin, and lidocaine patches may also be considered for neuropathic pain. Interventional approaches, including the use of corticosteroid injections, can add supplemental benefit.
Use of Opioids
Opioids should not be considered as initial treatment for acute or chronic pain. Nonpharmacologic treatments and nonopioid medications do not have to sequentially fail before initiating opioid therapy, but the expected benefits should be weighed against the risks.
STARTING OPIOIDS
When initiating opioids, the lowest effective dose should be used, especially for opioid-naive patients. This is usually a single dose of morphine equivalent, up to 10 mg, or a dosage of up to 30 mg daily of morphine equivalent (Table 2). Avoid rapid dosing increases because a risk of sedation, respiratory depression, and overdose can occur. Doses of more than 50 mg do not improve pain relief for most patients with noncancer pain, and overdose risk increases with dosing increases.
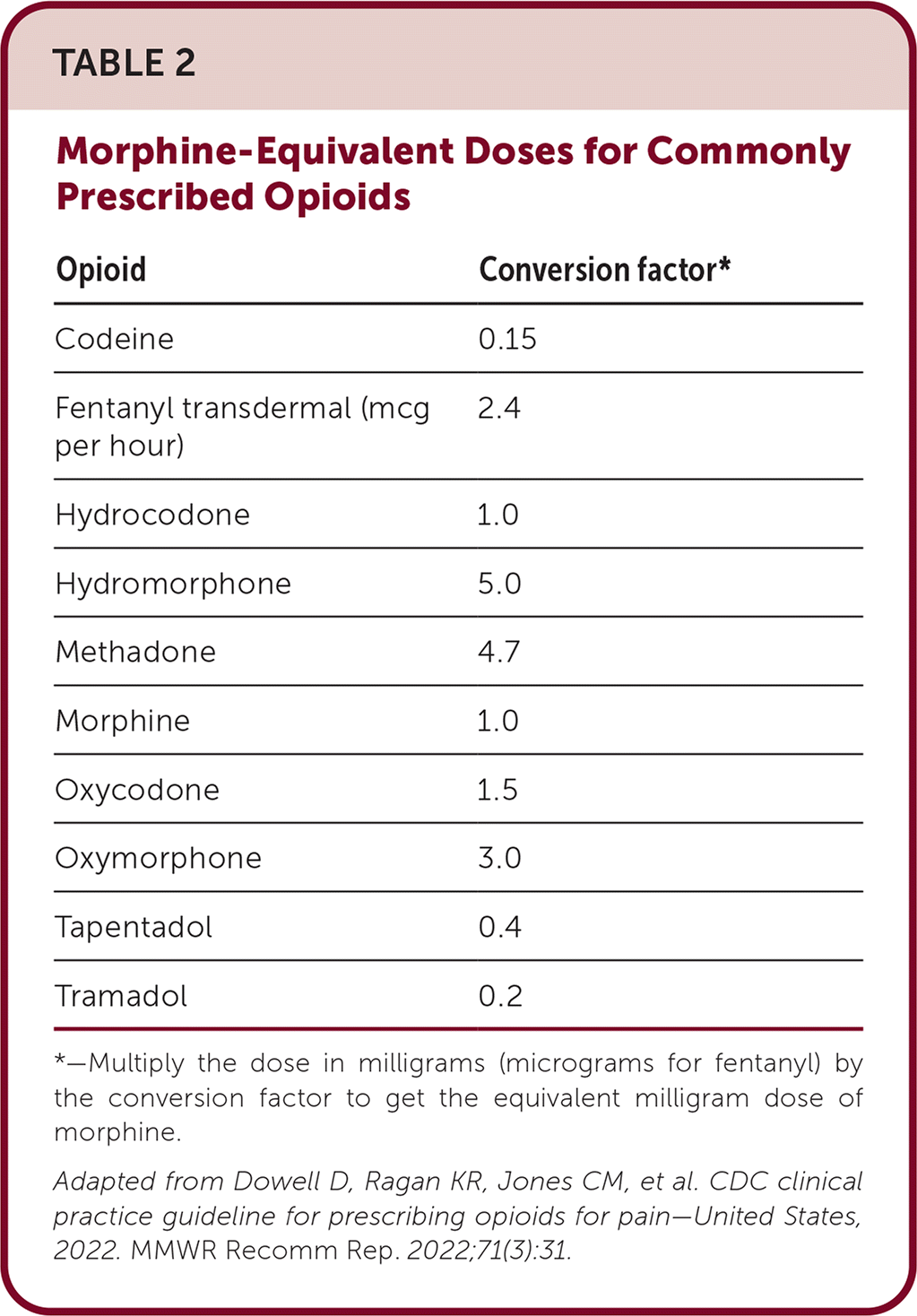
| Opioid | Conversion factor* |
|---|---|
| Codeine | 0.15 |
| Fentanyl transdermal (mcg per hour) | 2.4 |
| Hydrocodone | 1.0 |
| Hydromorphone | 5.0 |
| Methadone | 4.7 |
| Morphine | 1.0 |
| Oxycodone | 1.5 |
| Oxymorphone | 3.0 |
| Tapentadol | 0.4 |
| Tramadol | 0.2 |
Physicians should advise patients about the risks of opioid therapy, including fatal respiratory depression and opioid use disorder, and more common adverse effects, including constipation, dry mouth, nausea, drowsiness, confusion, tolerance, and withdrawal. An exit strategy should be considered if opioid therapy is unsuccessful. Physicians should determine the way in which functional benefits will be evaluated and establish measurable treatment goals.
For acute pain, opioids should be prescribed no more than needed for the expected duration of severe pain. Mechanisms must be available for reevaluating patients if pain lasts longer than expected. If opioids are continued for acute pain, patients should be evaluated at least every two weeks.
Using opioids for acute pain increases the risk of long-term use. If opioids are used continuously for more than a few days but less than one week, physicians should prescribe a brief taper to minimize withdrawal symptoms, such as reducing the daily dosage by one-half for two days.
OPIOID FORMULATIONS
Long-acting or extended-release opioids should not be used to treat acute pain or prescribed for intermittent use. Longer-duration formulations increase the risk of overdose, and they should be reserved for severe, continuous pain. Scheduled use of long-acting or extended-release opioids is associated with a higher average daily dosage. When changing from immediate-release formulations to extended-release or long-acting opioids, patients should start with a lower total daily dosage to account for incomplete opioid cross-tolerance. Methadone and fentanyl transdermal have unpredictable pharmacokinetics and should be prescribed only by physicians who have experience with these medications.
CONTINUING OPIOID THERAPY
Before initiating long-term (more than 30 days) opioid therapy, physicians should consider identifying reversible causes of pain and ensure an intentional decision of continuing long-term opioid therapy with shared decision-making based on benefits and risks. Rapid tapering or abrupt discontinuation of opioids should be avoided.
Physicians must carefully weigh benefits and risks before increasing opioid dosages for acute or continued pain. If benefits outweigh risks, nonopioid therapies must be optimized. If doses have been escalated for acute pain, physicians should return to the patient's baseline as soon as possible.
If benefits do not outweigh risks, a gradual taper should be initiated while other therapies are optimized. When a consensus cannot be reached, physicians should acknowledge the discordance, express empathy, and implement patient-centered treatment changes without abandoning the patient.
TAPERING OPIOIDS
A taper should be considered when a patient requests a lower dose, their pain improves, use has not improved pain or function, or the benefit-risk balance is unclear. Adverse effects, an increasing opioid dose, medical history, taking other medications, or a warning sign of an overdose may increase risk from opioid use.
Tapering can be harmful in some situations. When tapering opioids, it should be slow enough to minimize symptoms and signs of withdrawal (Table 3). For patients who have taken opioids for a year or more, tapers can take months to years. Slow tapers with a 10% reduction in the daily dosage per month or less are better tolerated. Withdrawal symptoms may signal that the taper needs to be slower. Tapers may have to be stopped and restarted at times. Tizanidine may be helpful in tapering patients from long-term, high-dose opioid use.
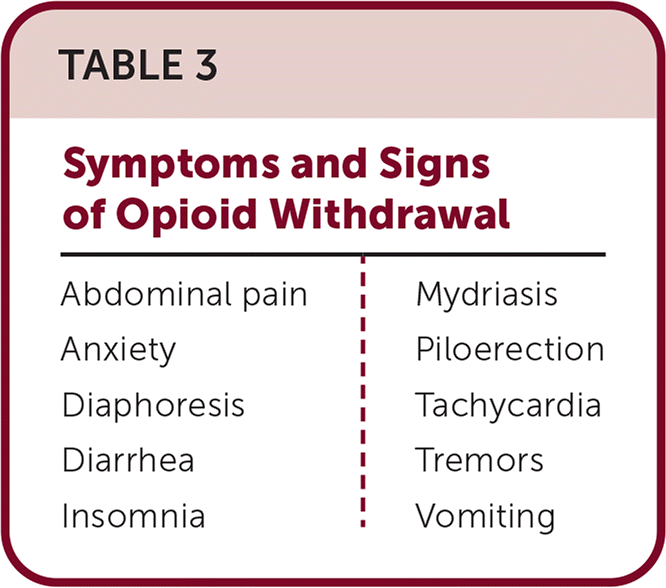
| Abdominal pain | Mydriasis |
| Anxiety | Piloerection |
| Diaphoresis | Tachycardia |
| Diarrhea | Tremors |
| Insomnia | Vomiting |
Nonopioid pharmacologic and nonpharmacologic treatments should be maximized while tapering. Anxiety, depression, and opioid use disorder may be revealed by an opioid taper, and studies suggest that follow-up should occur at least weekly.
Successful tapers can result in a lower dose or discontinuation of opioids. Transitioning to buprenorphine can reduce risk, even at equivalent dosages, but requires waiting at least eight hours after the last dose of short-acting opioids and 12 hours after extended-release or long-acting opioids.
LONG-TERM OPIOID THERAPY
Patients receiving long-term therapy should be assessed at least every three months for their perspective, progress toward treatment goals, risk of opioid use disorder or overdose, and the treatment of psychological comorbidities. Suggested goals for clinically meaningful improvement include a 30% improvement in pain and function.
In addition to looking for signs of overuse, screening for substance misuse, depression, and anxiety can be helpful. Physicians should review the state prescription drug–monitoring program to help assess risk, but results should not be used to dismiss patients. Urine toxicology testing can further assess risk, but results should not be used in a punitive manner.
Physicians should offer naloxone when prescribing opioids, particularly to patients at increased risk of overdose (i.e., history of overdose, taking other medications that increase risk, or having medical conditions that require higher opioid dosages). Naloxone education should be provided to household members.
| Score | Criteria |
|---|---|
| Yes | Focus on patient-oriented outcomes |
| Yes | Clear and actionable recommendations |
| Yes | Relevant patient populations and conditions |
| Yes | Based on systematic review |
| Yes | Evidence graded by quality |
| Yes | Separate evidence review or analyst in guideline team |
| Yes | Chair and majority free of conflicts of interest |
| Yes | Development group includes most relevant specialties, patients, and payers |
| Overall – useful |
The views expressed are those of the author and do not necessarily reflect the official policy or position of the Naval Undersea Medical Institute, U.S. Navy, U.S. Department of Defense, or U.S. government.
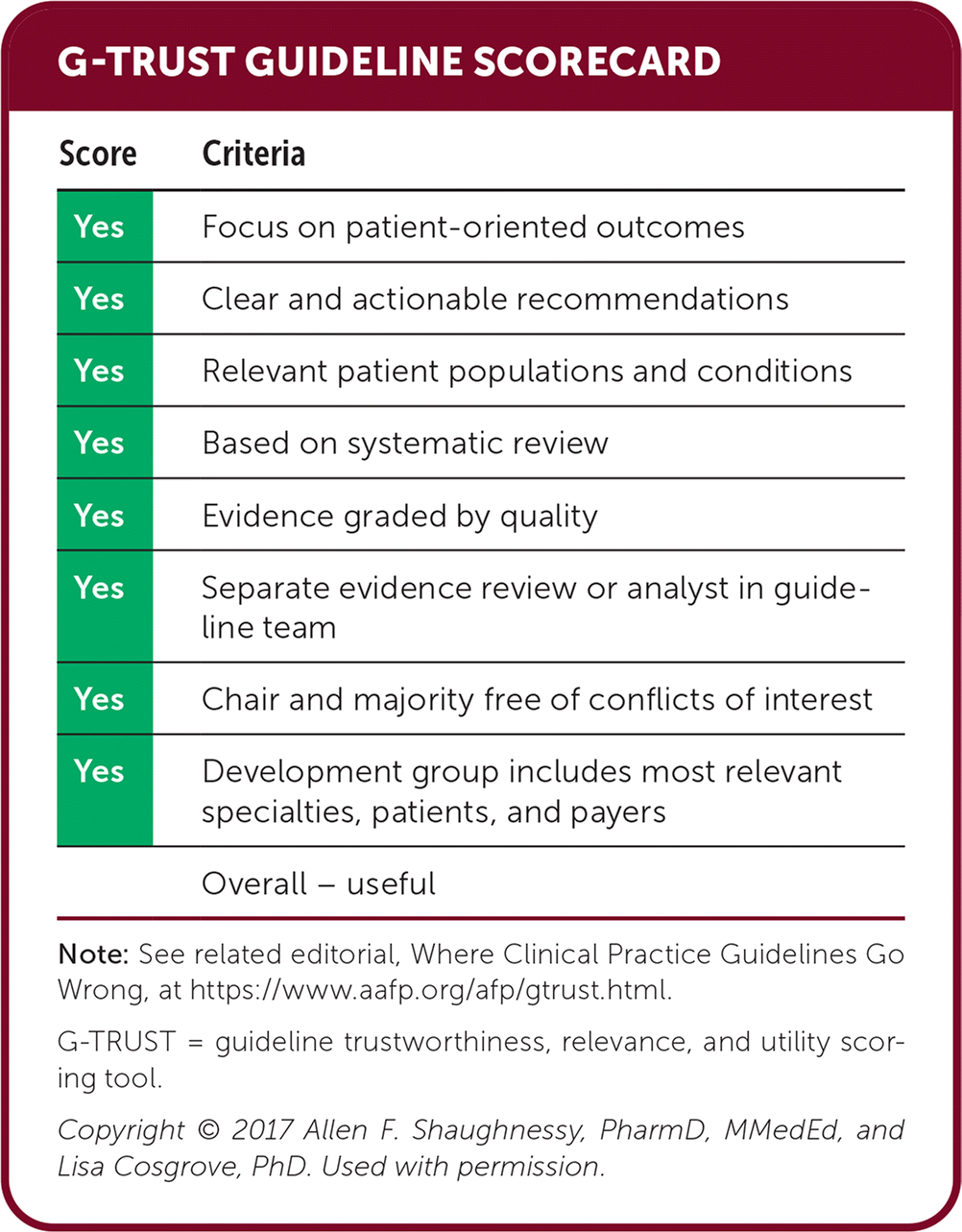
Editor's Note: This new CDC guidance for opioid prescribing is interesting in that it walks back the legacy of their own 2016 guidance. These guidelines are careful to avoid strict requirements and limit the role of dosing thresholds, urine toxicology, and the prescription drug monitoring–program. Many of us work under strict state requirements that are not aligned with this guidance. Recommendations for when and how to taper are more detailed than in previous CDC guidelines.—Michael J. Arnold, MD, Assistant Medical Editor
Guideline source: Centers for Disease Control and Prevention
Published source: Dowell D, Ragan KR, Jones CM, et al. CDC clinical practice guideline for prescribing opioids for pain — United States, 2022. MMWR Recomm Rep. 2022;71(3):1–95.
Available at: https://www.cdc.gov/mmwr/volumes/71/rr/rr7103a1.htm
