Am Fam Physician. 2024;109(1):88-89
Author disclosure: No relevant financial relationships.
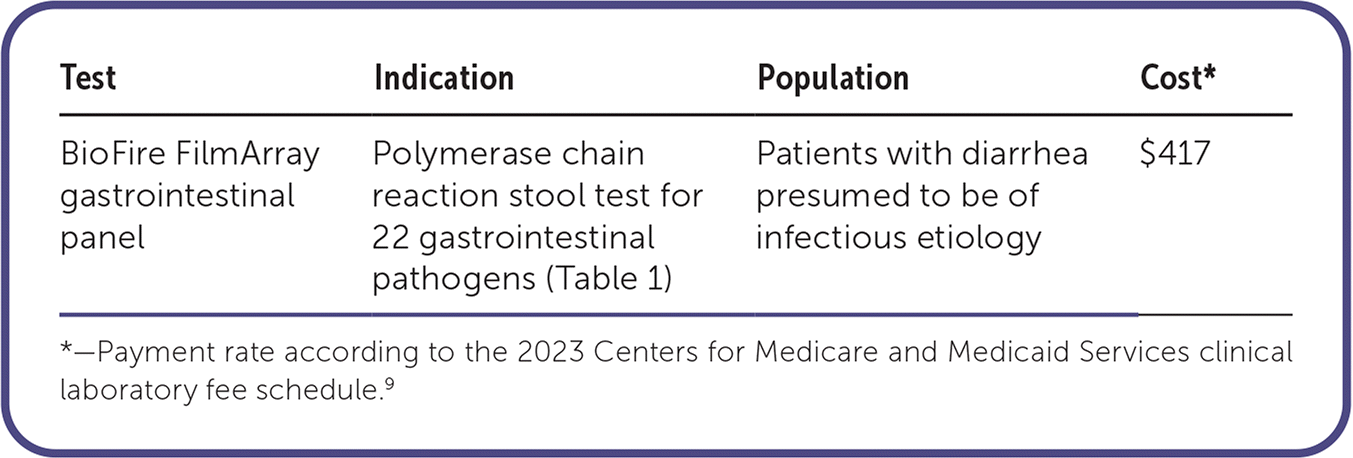
The BioFire FilmArray gastrointestinal (GI) panel is a multiplex polymerase chain reaction (PCR) test approved by the U.S. Food and Drug Administration to assess for 22 common viral, bacterial, and parasitic gastrointestinal pathogens.1 Stool culture, Clostridioides difficile toxin assay, or ova and parasite testing are typically reserved for patients with severe or prolonged disease, possible C. difficile infection, or unique risks for parasitic infection, respectively.2,3 In most patients with acute gastroenteritis, testing for a specific etiology is not performed, and confirmation of the pathogen does not change management of self-limited illness.
Accuracy
A multicenter study of stool specimens from 1,556 patients (62% younger than 21 years, 87% outpatient) compared the FilmArray GI panel with the results of conventional stool culture and molecular methods.4 Overall, the FilmArray GI panel was at least 94.5% sensitive and 97% specific for most panel targets.4 Individual pathogen accuracy is summarized in Table 1.4 In some cases, PCR detected a potential pathogen more often than the reference test.
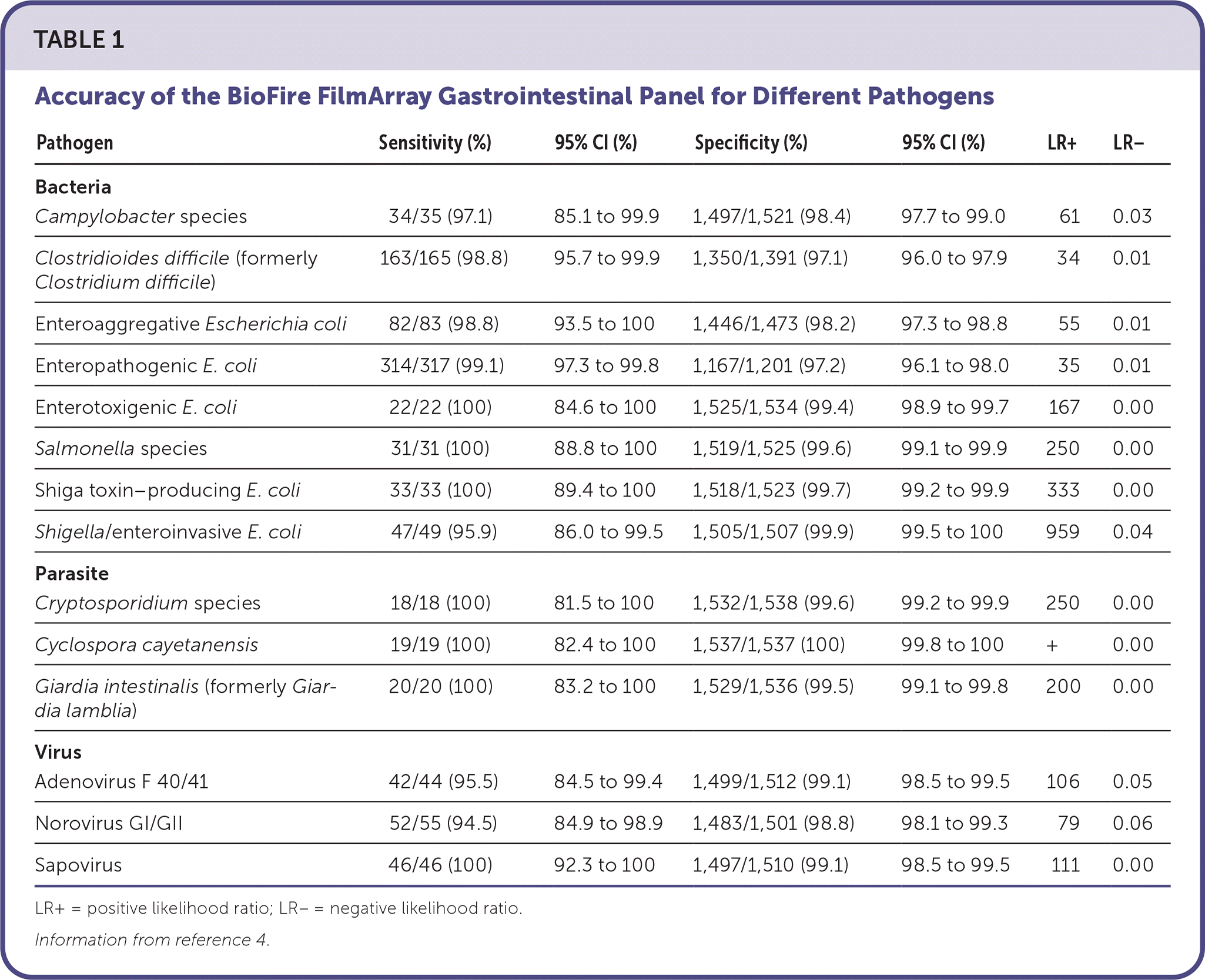
| Pathogen | Sensitivity (%) | 95% CI (%) | Specificity (%) | 95% CI (%) | LR+ | LR− |
|---|---|---|---|---|---|---|
| Bacteria | ||||||
| Campylobacter species | 34/35 (97.1) | 85.1 to 99.9 | 1,497/1,521 (98.4) | 97.7 to 99.0 | 61 | 0.03 |
| Clostridioides difficile (formerly Clostridium difficile) | 163/165 (98.8) | 95.7 to 99.9 | 1,350/1,391 (97.1) | 96.0 to 97.9 | 34 | 0.01 |
| Enteroaggregative Escherichia coli | 82/83 (98.8) | 93.5 to 100 | 1,446/1,473 (98.2) | 97.3 to 98.8 | 55 | 0.01 |
| Enteropathogenic E. coli | 314/317 (99.1) | 97.3 to 99.8 | 1,167/1,201 (97.2) | 96.1 to 98.0 | 35 | 0.01 |
| Enterotoxigenic E. coli | 22/22 (100) | 84.6 to 100 | 1,525/1,534 (99.4) | 98.9 to 99.7 | 167 | 0.00 |
| Salmonella species | 31/31 (100) | 88.8 to 100 | 1,519/1,525 (99.6) | 99.1 to 99.9 | 250 | 0.00 |
| Shiga toxin–producing E. coli | 33/33 (100) | 89.4 to 100 | 1,518/1,523 (99.7) | 99.2 to 99.9 | 333 | 0.00 |
| Shigella/enteroinvasive E. coli | 47/49 (95.9) | 86.0 to 99.5 | 1,505/1,507 (99.9) | 99.5 to 100 | 959 | 0.04 |
| Parasite | ||||||
| Cryptosporidium species | 18/18 (100) | 81.5 to 100 | 1,532/1,538 (99.6) | 99.2 to 99.9 | 250 | 0.00 |
| Cyclospora cayetanensis | 19/19 (100) | 82.4 to 100 | 1,537/1,537 (100) | 99.8 to 100 | + | 0.00 |
| Giardia intestinalis (formerly Giardia lamblia) | 20/20 (100) | 83.2 to 100 | 1,529/1,536 (99.5) | 99.1 to 99.8 | 200 | 0.00 |
| Virus | ||||||
| Adenovirus F 40/41 | 42/44 (95.5) | 84.5 to 99.4 | 1,499/1,512 (99.1) | 98.5 to 99.5 | 106 | 0.05 |
| Norovirus GI/GII | 52/55 (94.5) | 84.9 to 98.9 | 1,483/1,501 (98.8) | 98.1 to 99.3 | 79 | 0.06 |
| Sapovirus | 46/46 (100) | 92.3 to 100 | 1,497/1,510 (99.1) | 98.5 to 99.5 | 111 | 0.00 |
Benefit
Results are typically available in one to two hours, shortening the time to diagnosis when compared with traditional culture methods, which may take 24 to 48 hours or more. PCR-based tests are 1.5 times more likely to detect a pathogen than conventional testing due to higher sensitivity.5
Harms
Concurrent detection of multiple organisms with multiplex PCR panels is not uncommon (16% to 28% of samples), and the clinical implications of this are unknown.6 In one large series of 1,416 hospitalized patients tested for C. difficile using toxin immunoassay and PCR, 293 patients (21%) were positive by a PCR test, but only 131 (9%) were positive by a toxin immunoassay. Patients who had a negative toxin immunoassay result but a positive PCR result had similar duration of diarrhea as patients who tested negative on both tests, questioning the clinical significance of PCR detection.7 There is potential harm in the overtreatment of patients with nonpathogenic results.
Failing to consider noninfectious etiologies of chronic diarrhea or missing an infection caused by an untested organism may also cause additional harm.
Cost
The FilmArray GI panel is significantly more expensive than conventional testing. According to the Centers for Medicare and Medicaid Services, GI pathogen panels testing for 12 or more targets are medically reasonable and necessary only for patients with an immunocompromising medical condition presenting with acute or persistent diarrhea. Testing will not be covered in other patients.8 Commercial insurance is likely to follow the same guidance. The cost of the FilmArray GI panel is $417.9 In comparison, the cost of stool culture, stool ova and parasite testing, C difficile enzyme immunoassay, and C. difficile PCR ranges from $9 to $37 for each individual test.
Bottom Line
Multiplex PCR tests have the potential to shorten the time to diagnosis and increase the diagnostic yield for carefully selected patients presenting with infectious diarrhea. However, this testing comes at a significant cost that is often not covered by insurance, and the potential impact of this testing for patient management and outcomes is unclear. Family physicians should consider this test only for immunocompromised or critically ill patients and should continue to select well-studied and cost-effective tests for most patients, when indicated.