Am Fam Physician. 2024;109(2):161-166
Author disclosure: No relevant financial relationships.
Abnormal uterine bleeding is a common and bothersome symptom in people using hormonal contraception, and it can lead to discontinuation of reliable methods of contraception and unintended pregnancies. Clinicians should counsel individuals about the potential for abnormal bleeding at initiation of the contraceptive method. After considering and excluding other potential causes of abnormal uterine bleeding, clinicians can offer treatment options specific to each hormonal contraceptive method. This article includes algorithms to help clinicians treat abnormal uterine bleeding in people using levonorgestrel intrauterine devices, depo-medroxyprogesterone acetate, progestin implant, progestin-only pills, and combined hormonal contraception. For patients with levonorgestrel intrauterine devices, physicians should first ensure that the device is correctly placed within the uterus, then consider nonsteroidal anti-inflammatory drugs as a first-line treatment for abnormal uterine bleeding; estradiol can be used if nonsteroidal anti-inflammatory drugs are ineffective. For depo-medroxyprogesterone acetate or progestin implant users, combined oral contraceptives or nonsteroidal anti-inflammatory drugs may be considered. For patients using norethindrone progestin-only pills, changing to drospirenone progesterone-only pills may help reduce the bleeding. In people using combined hormonal contraception, it may be helpful to increase estrogen content from 20 mcg to 35 mcg per day, decrease the hormone-free interval (from seven to four or five days) in people using cyclic contraception, or start a trial of low-dose doxycycline. For continuous combined contraception users, adding a hormone-free interval of four or five days can help regulate bleeding patterns.
Abnormal uterine bleeding (AUB) is a common concern in individuals using hormonal contraception. However, before assuming AUB is due to hormonal contraception, alternative causes should be considered. The PALM-COEIN classification system is a helpful tool for evaluating potential underlying causes (Table 1).1 AUB caused by hormonal contraception falls under the iatrogenic category (i.e., the “I” in PALM-COEIN). Table 2 describes steps for further evaluation of AUB.1,2
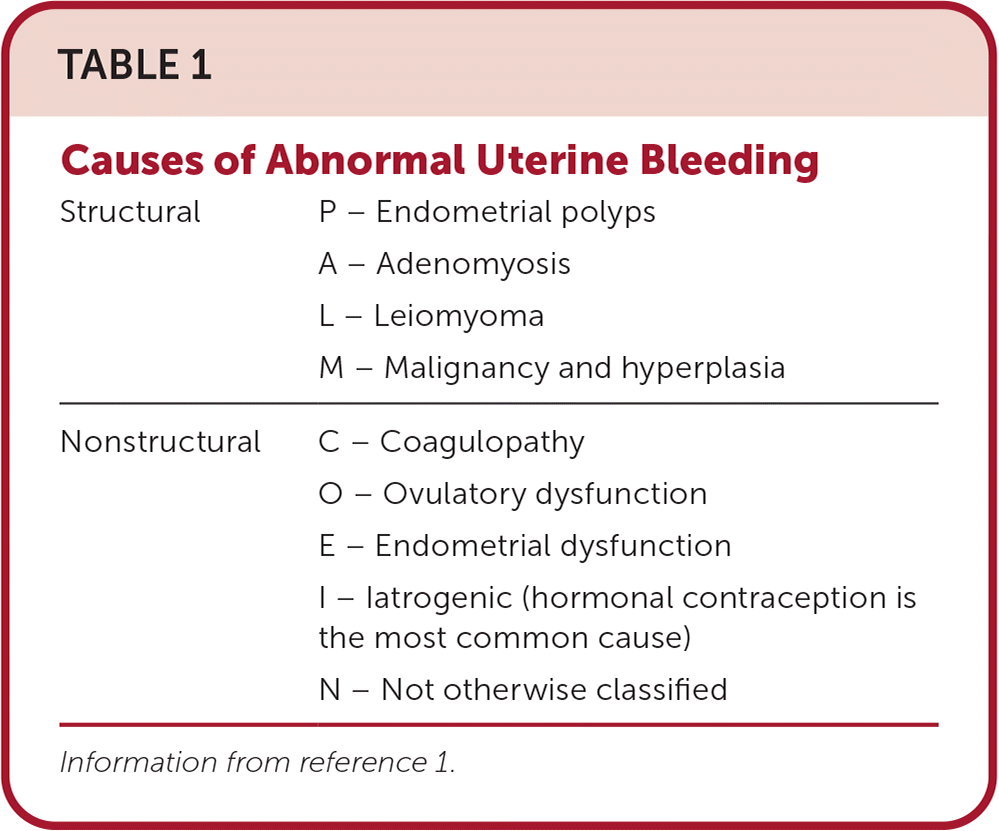
| Structural | P – Endometrial polyps |
| A – Adenomyosis | |
| L – Leiomyoma | |
| M – Malignancy and hyperplasia | |
| Nonstructural | C – Coagulopathy |
| O – Ovulatory dysfunction | |
| E – Endometrial dysfunction | |
| I – Iatrogenic (hormonal contraception is the most common cause) | |
| N – Not otherwise classified |

| For all methods Rule out pregnancy Review whether patients are using their chosen hormonal contraception method reliably; if they are missing doses, counsel them about setting phone reminders, using medication reminder apps, and asking for support from a family member or partner Inquire about recent use of exogenous hormones (e.g., gender-affirming hormone therapy, emergency contraception) Ensure that cervical cancer screening is up to date Consider screening for sexually transmitted infection Consider other etiologies; more information is available in a previous American Family Physician article (https://www.aafp.org/pubs/afp/issues/2019/0401/p435.html) Inquire about any medication changes, particularly those that might induce metabolism of estrogen (e.g., carbamazepine, azithromycin, rifampin) |
| For levonorgestrel intrauterine devices Confirm device placement with a pelvic examination and string check; if necessary, perform ultrasonography to ensure placement within the uterus |
The condition can also cause dissatisfaction with the contraceptive method of choice, leading to early discontinuation.3 One survey found that up to 42% of women had discontinued hormonal contraception due to dissatisfaction, and AUB was the second most common reason for discontinuation.4 The potential for AUB should be discussed with the patient before initiation of any hormonal contraception.
Educating the patient about the expected duration of bleeding and therapeutic options can aid shared decision-making to find an acceptable contraception plan and improve continuation rates. Among patients using levonorgestrel intrauterine devices (LNG-IUDs), 35% may experience frequent or prolonged bleeding within the first three months of use; however, this is likely to decrease within 12 months of use.5 Additionally, 90% of LNG-IUD users will have a decrease in bleeding within 12 months and 17% to 20% will experience amenorrhea within 12 months.3 With the progestin subcutaneous implant, up to 23% of users may experience frequent or prolonged bleeding, which is less likely to improve with time.6 Depo-medroxyprogesterone acetate (DMPA) may cause longer menses in 26% of users in the first three months. However, this improves significantly with continued use; roughly 85% of patients have symptom resolution by 12 months.7,8 AUB affects 30% to 50% of combined hormonal contraception (CHC) users during the first three to six months.9 After three months of using cyclic CHC, the rate of AUB improves to about 10% of patients.9
Irregular bleeding can be a frustrating experience. It is important for clinicians to validate patient concerns and affirm the patient's agency over the treatment plan. The recommendations and algorithms in this article are intended to guide physicians using the available evidence. Given the lack of research on this topic, recommendations are often based on few limited studies.
Levonorgestrel IUD
The etiology of AUB with the LNG-IUD is not clearly understood.10 Light menses before insertion may predict a more favorable bleeding pattern with LNG-IUD use.11 One small study on LNG-IUD position, as visualized with ultrasonography, found that individuals who had intrauterine nonfundal placement, instead of placement less than 0.5 cm from the fundus with both arms extended, had higher rates of bleeding after six months of use.12 Nonfundal placement was seen in roughly 70% of individuals after insertion, but in only about 30% of individuals after six months of use.
Most studies on the treatment and prevention of AUB associated with LNG-IUDs have used only the 52-mg LNGIUD. After insertion of the device, AUB was less severe in patients taking 500 mg of naproxen twice daily for five days at four-week intervals over 12 weeks.13 Evidence regarding the role of estradiol to control AUB due to LNG-IUD use is mixed—although the use of estradiol patches for the first 12 weeks after insertion can increase irregular bleeding,13 a more recent small study using oral estradiol (2 mg daily for six weeks) showed a significant reduction in the number of irregular bleeding days14 (Figure 112–17). A 100-mg dose of mifepristone, taken once on the day of insertion and repeated once every 30 days for three cycles, resulted in fewer bleeding days and increased satisfaction with the LNG-IUD. However, high cost and limited availability of mifepristone also make it unfeasible for many physicians based in the United States.17 Interventions such as timing the insertion early in the menstrual cycle and administering mifepristone before insertion have not been shown to improve AUB.18,19 One study showed that tranexamic acid does not significantly improve long-term bleeding patterns.20
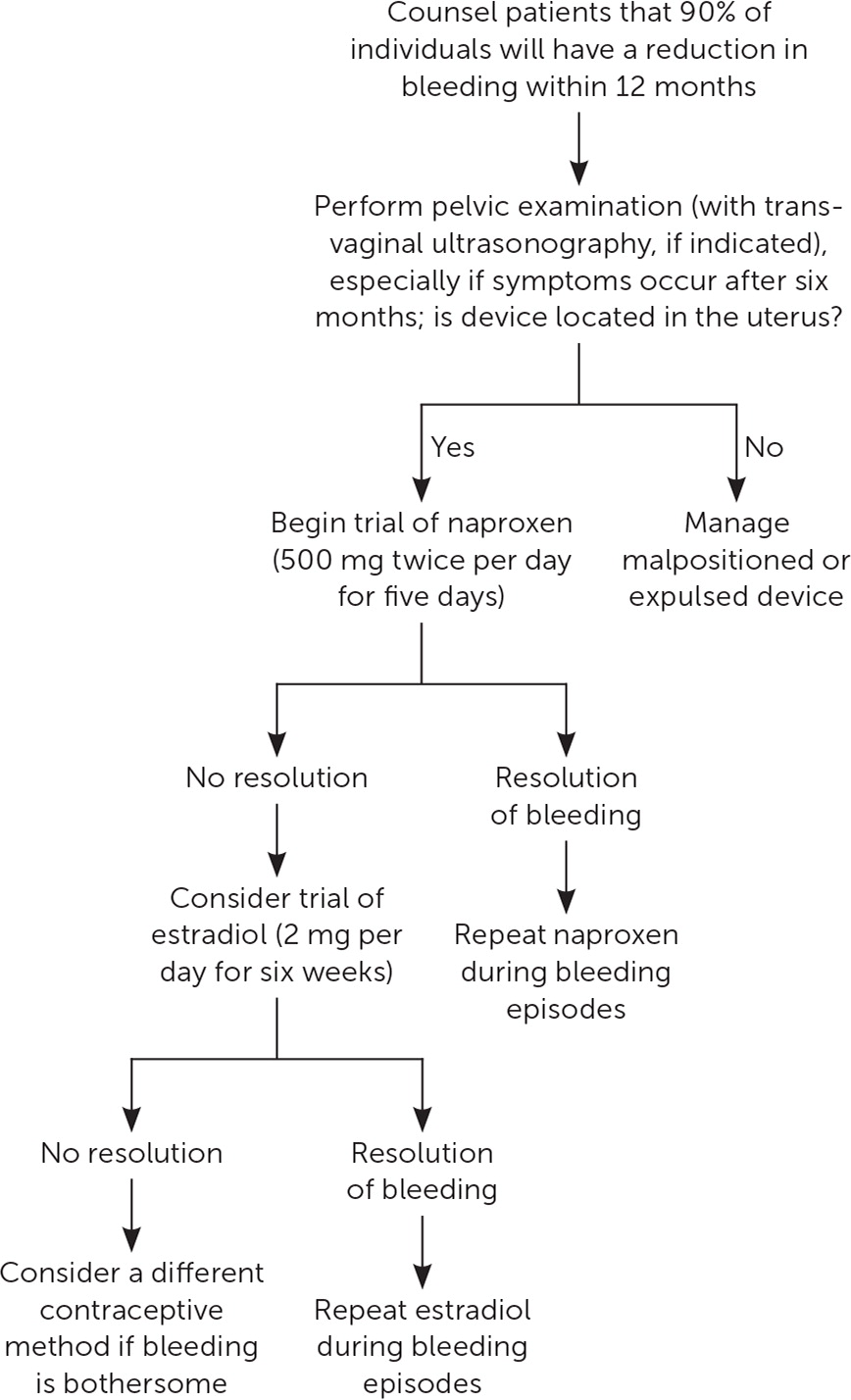
Contraceptive effectiveness and favorable bleeding patterns have been demonstrated for up to eight years with the 52-mg LNG-IUD.15 After placing a second 52-mg LNG-IUD following five years of use, AUB rates increase initially, then improve to even lower rates than before replacement.16 For patients experiencing AUB or noticing an increase in bleeding after five years with the 52-mg LNG-IUD, it is reasonable to consider removal and replacement.
Depo-medroxyprogesterone Acetate, Progestin Implant, Progestin-Only Pills
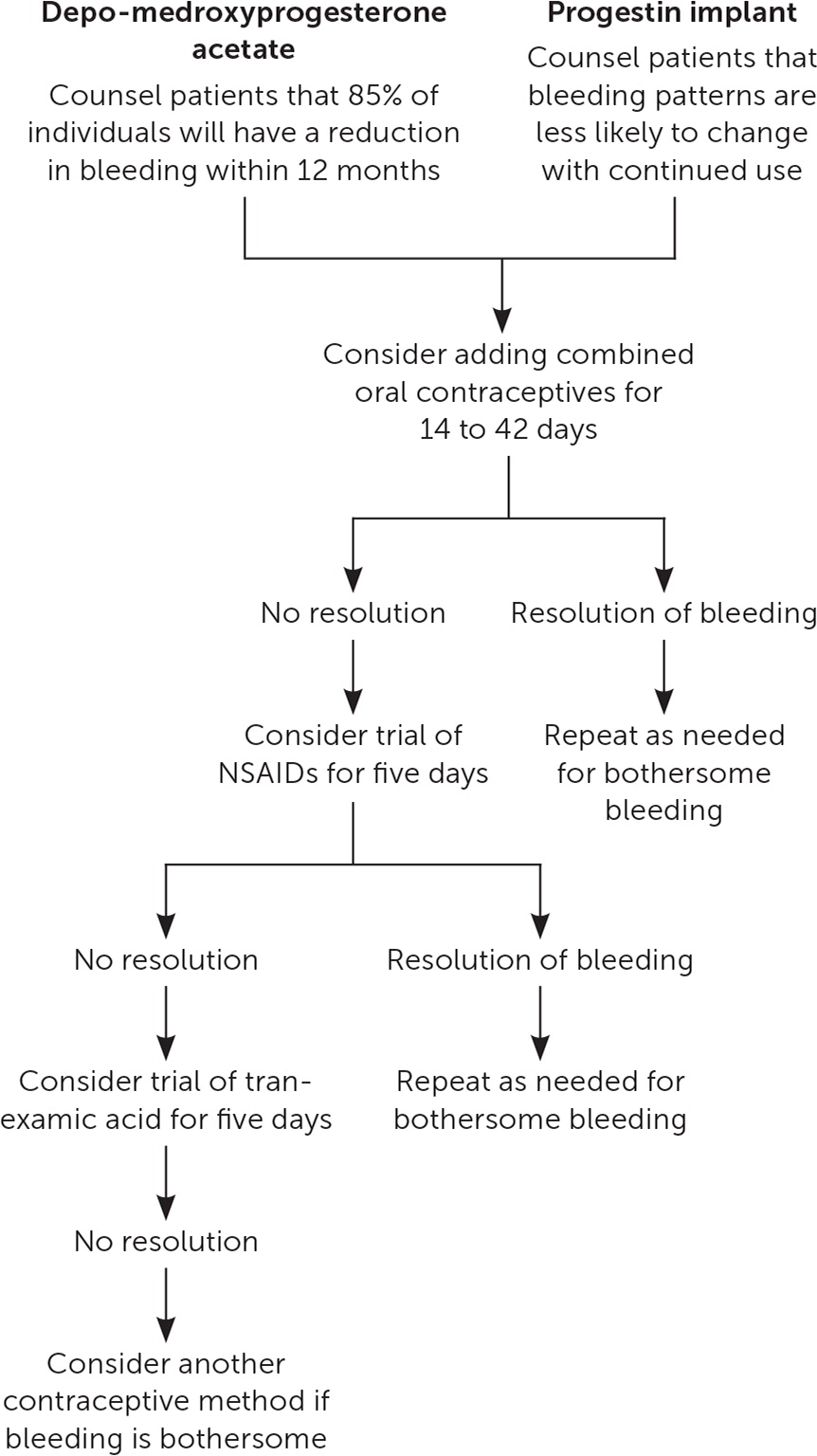
Depo-medroxyprogesterone acetate (DMPA) users are more likely to experience amenorrhea with continued use, whereas irregular bleeding with the progestin implant or norethindrone progestin-only pills (POPs) is less likely to self-resolve. When DMPA users experience AUB after initiation, watchful waiting is a reasonable option. Many cases of DMPA-induced AUB will improve after six months (two injections), and most cases will resolve after one year.7,8,16 With the progestin implant, bleeding patterns vary widely and can be unpredictable. After the first three months of use, the observed bleeding pattern is unlikely to change with additional time.6
For DMPA and progestin implant users who desire a quick solution for irregular bleeding, limited evidence supports the use of combined oral contraceptives, nonsteroidal anti-inflammatory drugs (NSAIDs), or tranexamic acid. Of these methods, combined oral contraceptives may offer the fastest resolution,21 but medical contraindications to estrogen therapy may limit its use. Treatment with a combined oral contraceptive containing 20 to 30 mcg of ethinyl estradiol once per day for two or six weeks improves the number of irregular bleeding days in most progestin implant users.21–23 Once the combined oral contraceptive is stopped, irregular bleeding patterns may return.
When estrogen therapy is contraindicated, NSAIDs or tranexamic acid can be considered for treatment of irregular bleeding.24–27 Mefenamic acid (500 mg twice per day for five days) is the only NSAID that has been studied for AUB related to DMPA and progestin implants.21,24,27 Because mefenamic acid may be expensive and is not often prescribed in the United States, more common NSAIDs such as ibuprofen (800 mg three times per day) or naproxen (500 mg twice per day for five days) could be considered. Based on small studies, AUB related to DMPA or progestin implants is more likely to resolve one week after completion of treatment, but improvement may no longer be seen four weeks after completion of treatment.24,27 Tranexamic acid is an antifibrinolytic agent used for treatment of menorrhagia. For DMPA and progestin implant users without a history of venous thromboembolism, tranexamic acid (500 mg twice per day for five days) appears to improve AUB after one week, but the benefit after four weeks is uncertain.25,26
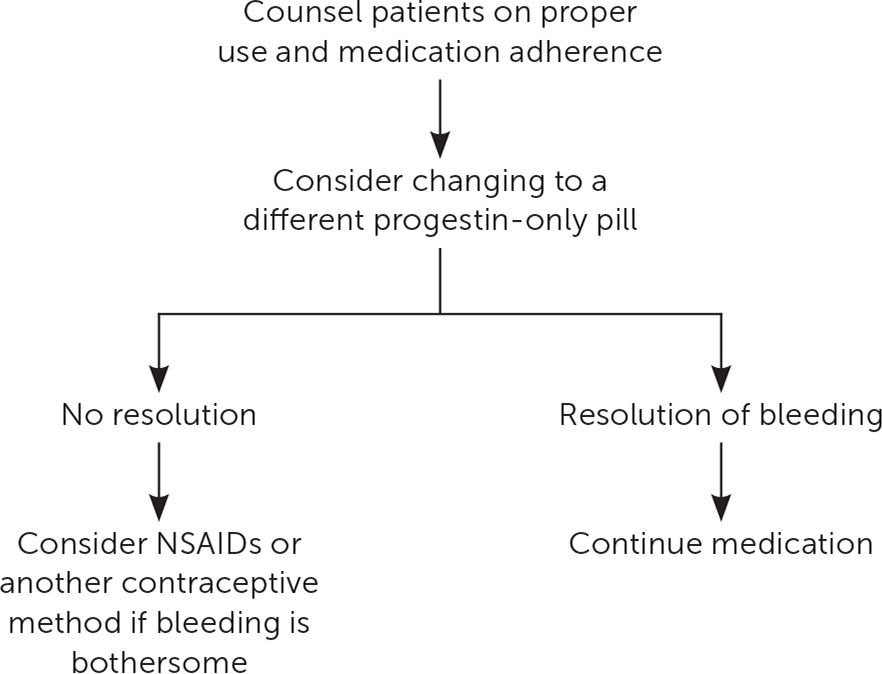
Historically, progestin-only pills (POPs) have been associated with a higher rate of AUB compared with oral contraceptives containing both estrogen and progesterone, leading to compliance issues and higher failure rates.28 In POP users with AUB, it would be reasonable to switch to a POP with a more favorable bleeding profile. Drospirenone POPs (Slynd) are offered in a dosing regimen of 24 active pills and four inactive pills. Drospirenone POPs have been associated with less unscheduled bleeding compared with other POPs.29,30 For the only over-the-counter norgestrel POP (Opill), the bleeding profile and how it compares with other POPs are still uncertain.
Combined Estrogen/Progestin Methods
Combined hormonal contraceptives (CHCs) include combined oral contraceptives, the contraceptive ring (Nuvaring), and the contraceptive patch. In CHCs, the progestin component provides most of the contraceptive benefit, whereas the estrogen component provides endometrial stability and cycle control.28,31 Early oral contraceptives contained 150 mcg of ethinyl estradiol and demonstrated excellent cycle control but had high rates of adverse effects, such as venous thromboembolism, nausea, and breast tenderness. The 35 mcg of ethinyl estradiol or less in most current CHCs reduce these significant adverse effects but cause higher rates of AUB.31 Use of oral contraceptives with 10 or 20 mcg of ethinyl estradiol leads to more bleeding complications and discontinuation compared with use of 30-mcg ethinyl estradiol pills,32 although differences in the progestin component of the pills may also contribute. Therefore, increasing estrogen doses in patients with AUB or changing to a formulation with a different progestin is a reasonable option.
Patients may choose to use their CHC continuously (i.e., without a monthly withdrawal bleed) due to ease of use. CHC can also be taken in an extended cycle (i.e., more than 28 days of active hormones, followed by a scheduled hormone-free interval).33 Continuous and cyclic CHC use results in similar contraceptive effectiveness and adherence, whereas continuous use may lead to more favorable bleeding profiles.33,34 There is no difference in AUB rates in women taking monophasic pills compared with triphasic pills.35 Adding a four- or five-day hormone-free interval in people using continuous contraception who experience AUB for at least five to seven days is effective.36–38 Shortening the hormone-free interval from seven days to four or five days in people using cyclic CHC may reduce menstrual symptoms by decreasing the number of bleeding days39 (Figure 432,36–42).
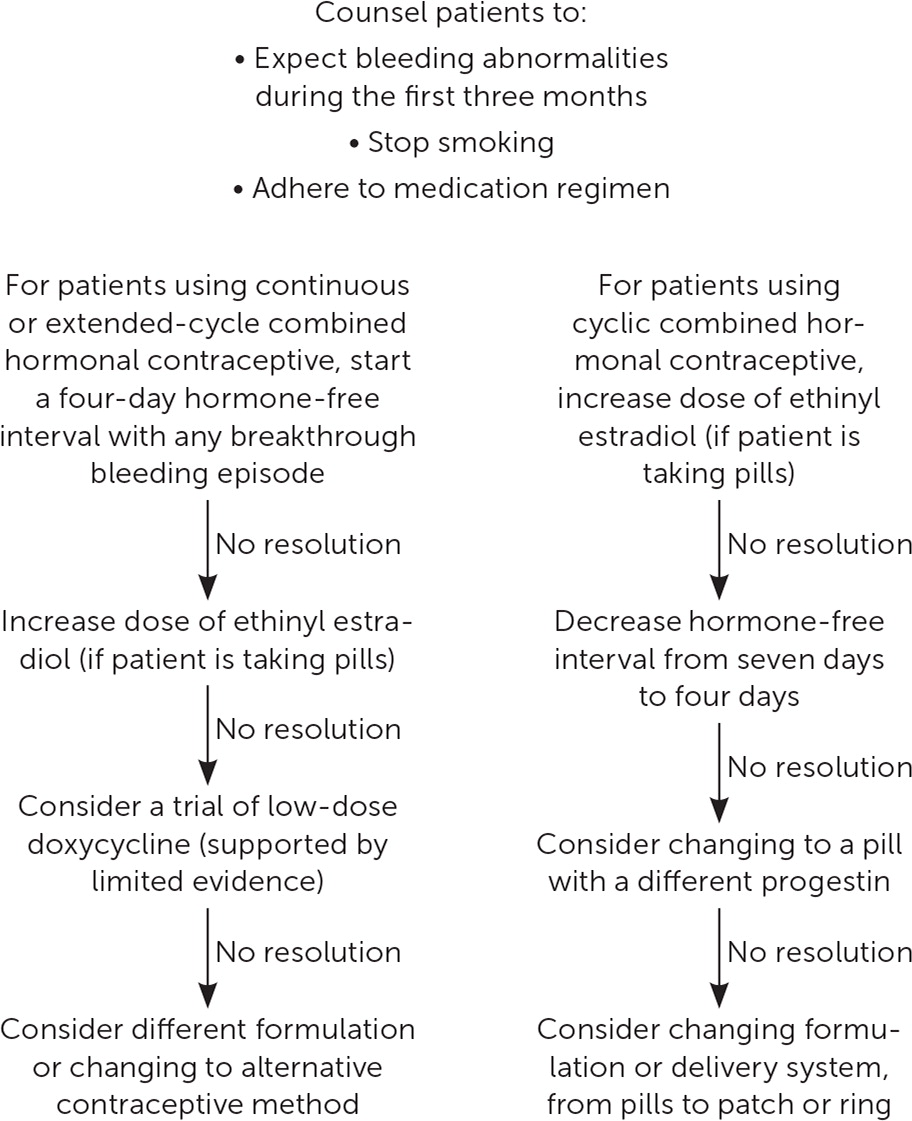
After the first three months of combined oral contraceptive use, missed pills are the most common cause of AUB. Smoking can also reduce the reliability of medication absorption and precipitate AUB.9
The contraceptive patch and ring are similarly effective compared with oral contraceptives and may have more favorable bleeding profiles.40 However, when AUB presents with the patch or ring, adjustment of the estrogen dose or type of progestin is not possible.
AUB associated with hormonal contraception may continue despite these treatments, but physicians can reassure patients that bleeding can be monitored or the contraceptive method discontinued. Close follow-up to discuss options can help prevent gaps in contraception and unintended pregnancy.
Data Sources: A PubMed search was completed by a reference librarian in Clinical Queries using the key terms hormonal contraception, levonorgestrel IUD, abnormal bleeding, depomedroxyprogesterone acetate, combined hormonal contraception, progestin only pills, and nexplanon. The search included meta-analyses, randomized controlled trials, clinical trials, and reviews. We also searched the Agency for Healthcare Research and Quality evidence reports, Clinical Evidence, the Cochrane database, Essential Evidence Plus, and DynaMed. Search date: November 20, 2022.